Chapter 46 Candidiasis
INTRODUCTION
Oropharyngeal candidiasis (OPC) was among the initial manifestations recognized in association with human immunodeficiency virus (HIV) infection,1,2 affecting most persons with advanced untreated HIV infection. Its importance is often obscured by the occurrence of other severe opportunistic infections seen with acquired immunodeficiency syndrome (AIDS). OPC may be a sentinel event for the detection or progression of HIV disease, presenting months or years before more severe opportunistic disease.3–5
Although usually associated with slight morbidity, OPC can be clinically significant. Severe OPC can interfere with the administration of medications and adequate nutritional intake and may extend to involve the esophagus.6 Symptoms may include burning pain, altered taste sensation, and difficulty swallowing liquids and solids. Many patients are asymptomatic. Pseudomembranous candidiasis, or thrush (white plaques on the buccal mucosa, gums, or tongue), is the most common presentation for OPC. Less commonly persons have acute atrophic candidiasis (erythematous) or chronic hyperplastic candidiasis (leukoplakia) involving the tongue.
Esophageal candidiasis (EC) is usually accompanied by the presence of OPC. Typically, dysphagia and odynophagia are described. Esophageal involvement is asymptomatic in as many as 40% of patients with OPC.6 Esophageal disease occasionally presents in the absence of clinically detectable oropharyngeal disease.
Invasive candidiasis typically occurs in persons with traditional risk factors (e.g., indwelling venous catheters, broad-spectrum antibacterials).7,8 Bloodstream infections, meningitis, intraabdominal infections, and osteomyelitis have been described, and the clinical manifestations of invasive candidiasis are similar to those of HIV-seronegative persons.
TAXONOMY AND BIOLOGY
Yeasts are fungi that grow as single cells and reproduce by budding. They are distinguished on the basis of the presence or absence of capsules, the size and shape of the yeast cells, the mechanism of daughter formation, the formation of true or pseudohyphae, and the presence of sexual spores, along with physiologic data. Candida albicans is the predominant causative agent of all forms of mucocutaneous candidiasis. Less frequently, C. glabrata, C. parapsilosis, C. tropicalis, C. krusei, and several other species cause disease. More recently, C. dubliniesis has been identified as a separate species distinct from C. albicans.9,10
Candida species are normal inhabitants of the human gastrointestinal tract and may be recovered from the mouths of up to one-third of normal individuals and two-thirds of those with advanced HIV disease.11,12 Colonization with an inherently resistant organism is more common in advanced HIV infection (CD41 T-lymphocyte counts of <50 cells/mm3).12 Most disease is caused by endogenous organisms of the individual, although rare cases of person-to-person transmission have been documented.13
MICROBIOLOGY
Candida strains affecting persons with HIV infection are typically no different from those in other immunosuppressed hosts.14 Candida dubliniesis is more commonly identified in HIV-infected persons, though its clinical significance is, at this point, indistinguishable from that of C. albicans.9,10 There are no detectable differences in the virulence of strains isolated from HIV-infected or HIV-uninfected persons. Recurrent disease usually results from the same species or strains of Candida,14–17 and the emergence of different strains or species is more likely in persons exposed to antifungal therapy with low CD4+ T-lymphocyte counts.18
EPIDEMIOLOGY
Mucocutaneous candidiasis occurs in three forms among persons with HIV infection; oropharyngeal, esophageal, and vulvovaginal disease. Oropharyngeal and vulvovaginal disease are the most common forms. Historically, up to 90% of persons with advanced untreated HIV infection developed OPC, with 60% having at least one episode per year with frequent recurrences.19–26 EC occurs less frequently (10–20%), but is the leading cause of esophageal disease.27–29 Vaginal candidiasis has been noted in 27–60% of women, similar to the rates of oropharyngeal disease.30–32 However, the incidence appears to be similar in HIV-infected and HIV-uninfected women.33,34 It is notable that 75% of all women of childbearing age develop vaginal candidiasis, and 40% have a second occurrence. Few women (,5%) experience frequent recurrences (defined as four or more infections during a 12-month period).
Two factors have affected the epidemiology of mucocutaneous candidiasis. The first was the widespread use of antifungal agents, particularly the azoles. Continuous use of azoles led to a decline in the prevalence of mucosal candidiasis while leading to the emergence of refractory infections. More importantly, the introduction of highly active antiretroviral therapy (HAART) resulted in a significant decline in the incidence of a number of opportunistic illnesses (e.g., Pneumocystis jirovecii pneumonia and cytomegalovirus infection).35–37 In turn, the incidence of mucocutaneous forms of candidiasis declined precipitously. For example, Cauda et al reported a significant difference in the incidence of recurrent OPC in patients treated with protease inhibitors compared with those not treated with protease inhibitors (7% vs 36%).38 Similarly, Martins et al reported a decline in the incidence of OPC from 30% to 4% over a 1-year period in persons on HAART.39
A number of factors are important in the development of mucocutaneous candidiasis. The level of immunosuppression is paramount.22 Other host factors important to the defense against Candida infections include blood group secretor status, salivary flow rates, epithelial barrier, antimicrobial constituents of saliva, the presence of normal bacterial flora, and local immunity.23,40 Several studies suggest impairment of a number of anti-Candida host defense mechanisms in persons with HIV infection.22,24,25 High levels of HIV-1 RNA in the plasma have been associated with increased rates of mucocutaneous candidiasis and colonization with Candida.41,42 It is notable that the relationship between the level of immunosuppression and vaginal candidiasis may not be as strong. In one cross-sectional study of 833 HIV-infected and 427 HIV-uninfected women the annual incidence of vaginal candidiasis was similar in the two groups (9%).33 There are few studies describing the incidence and prevalence of nonesophageal invasive candidiasis in HIV-infected persons. The incidence is probably less than 1%.7,8
DIAGNOSIS
Clinical Appearance
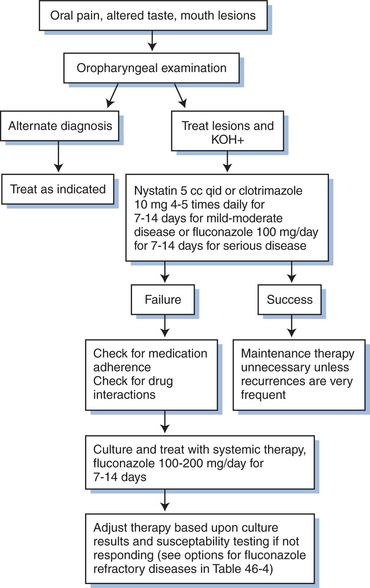
The diagnosis of OPC is usually made by its characteristic clinical appearance (Fig. 46-1). Recovery of an organism is not critical to the diagnosis of OPC, as oropharyngeal cultures often demonstrate Candida species, but frequently represent colonization.19 The diagnosis of OPC can be confirmed by examining a 10% KOH slide preparation of a scraping of an active lesion, which demonstrates characteristic pseudohyphae and budding yeast. A KOH preparation is simple to perform but not mandatory for diagnosing OPC. A presumptive diagnosis of OPC can be made by visual detection of characteristic lesions, with resolution of those lesions in response to antifungal therapy. In patients with poorly responsive OPC, a culture should be obtained to look for drug resistant yeasts or those that respond poorly to certain azoles (e.g., C. kruseii or C. glabrata). Biopsies of oral lesions are rarely helpful or indicated.
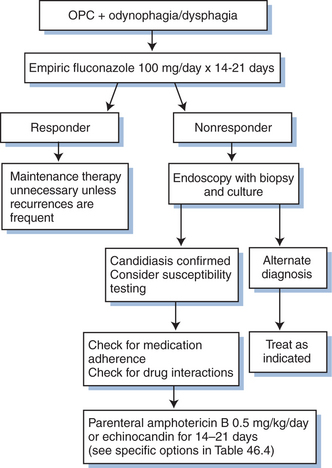
A presumptive diagnosis of Candida esophagitis is appropriate in a patient with dysphagia or odynophagia (or both) who has OPC (Fig. 46-2). Upper endoscopy can be used to confirm esophageal involvement; a barium swallow test may be used, but it is less sensitive and specific than endoscopy. These studies are not uniformly required unless a patient fails to improve with appropriate systemic antifungal therapy.29 If a patient with OPC does not resolve the esophageal symptoms despite resolution of the oral lesions, endoscopy is indicated to exclude other causes of esophagitis (e.g., CMV, herpes simplex virus, aphthous ulcers). The diagnosis of Candida esophagitis is confirmed by the presence of yeast forms on histologic examination of esophageal lesions. Specimens for culture should be obtained from patients who require endoscopy to look for drug-resistant Candida.
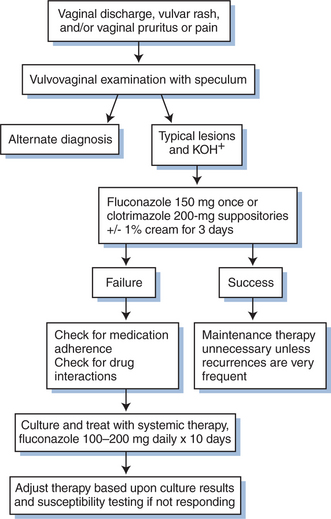
Candida vaginitis is diagnosed based on the presence of a characteristic clinical appearance and observation of yeast forms on a microscopic examination (Fig. 46-3). A KOH preparation should always be done on vaginal lesions to confirm the diagnosis of candidiasis because there are a number of other conditions that appear similar (e.g., trichomoniasis). Routine cultures are rarely helpful in the absence of KOH-positive lesions because yeasts are normal inhabitants of the vaginal mucosa. As for OPC, a culture should be prepared if a patient fails to respond to standard antifungal therapy. Vulvar disease is typically diagnosed by its characteristic appearance.
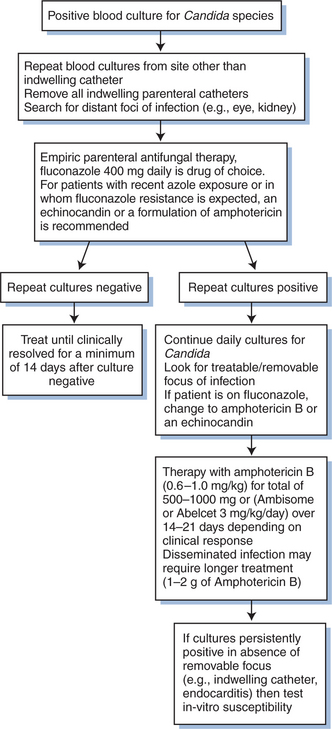
Invasive disease is diagnosed by demonstrating the presence of Candida from a sterile site (e.g., blood, bone biopsy, cerebrospinal fluid). Bloodstream infections are diagnosed when one or more cultures are positive for Candida (Fig. 46-4). In most clinical circumstances, a single positive blood culture for Candida should be considered significant.
Drug Resistance and Susceptibility Testing
Antifungal susceptibility testing is gradually becoming standardized. The Clinical Laboratory Standard Institute (CLSI) has definitions for in vitro susceptibilities for selected agents using standard methodologies.43 The most common methods for in vitro testing are macrotube and microtiter broth dilution assays, disk diffusion tests, and the E-test. In vitro susceptibilities should not be used routinely to guide the choice of antifungal agents, but rather to test isolates that are clinically refractory to antifungal therapy.
There are several mechanisms that explain in vitro resistance to antifungal drugs, including target alteration, reduced cell permeability, and active efflux of the drug out of the cell.44–47 Some yeasts have single drug resistance, whereas others are multidrug-resistant. Azole resistance has been demonstrated in yeasts containing alterations in the enzymes that were the target of their action or were involved in ergosterol biosynthesis. The relative incidence of the various mechanisms of resistance is unknown. Furthermore, it is not clear whether certain mechanisms of resistance may be overcome by higher dosing of the drug.
THERAPY
Numerous agents are effective against candidiasis (Table 46-1). Important factors that determine clinical response in addition to the choice of antifungal agent include the extent and severity of disease, patient adherence to the regimen, and the pharmacodynamic/pharmacokinetic properties of the drug. Treatment of OPC and vaginal candidiasis is relatively simple, with most types responding to therapy. Overall, there have been few clinical differences in randomized studies comparing topical with systemic therapy. Mild OPC or vulvovaginal disease can often be treated successfully with topical therapy. Moderate and severe episodes typically require systemic therapy. Esophagitis and other invasive disease always require systemic therapy.
Table 46-1 Therapeutic Options for Mucosal Candidiasis
| Medication | Dosage | Important Toxicities |
|---|---|---|
| Oropharyngeal Candidiasis | ||
| Clotrimazole troches | 10 mg 4–5 times/day × 7–14 days | Altered taste, GI upset |
| Nystatin suspension | 100 000 units/cm3 5 cc qid × 7–14 days | GI upset |
| Ketoconazole | 200 mg/day × 7–14 days | GI upset, hepatitis, endocrine effects |
| Itraconazole | 100 mg/day × 7–14 days | GI upset, hepatitis |
| Fluconazole | 100 mg/day × 7–14 days | GI upset, hepatitis |
| Esophageal Candidiasis | ||
| Fluconazolea | 100 mg/day × 14–21 days | GI upset, hepatitis |
| Ketoconazole | 400 mg/day × 14–21 days | GI upset, hepatitis, endocrine effects |
| Itraconazole | 200 mg/day × 14–21 days | GI upset, hepatitis |
| Parenteral amphotericin B | 0.5 mg kg−1 day−1 × 14–21 days | Renal failure, electrolyte losses, fever, chills, sweats |
| Lipid formulations of amphotericin B | 3 mg kg−1 day−1 × 14–21 days | Fever, chills, sweats, electrolyte losses, renal insufficiency uncommon |
| Voriconazole | 200 mg bid × 14–21 days | GI upset, hepatitis, visual impairment |
| Caspofungin | 50–70 mg/day × 14–21 days | Fever, infusion-associated phlebitis |
| Micafungin | 75–150 mg/day × 14–21 days | Fever, infusion-associated phlebitis |
| Andidulafungin | 50–100 mg/day × 14–21 days | Fever, infusion-associated phlebitis |
| Vulvovaginal Candidiasisb | ||
| Fluconazole | 150 mg once | Minimal GI upset |
| Butoconazole | ||
| 2% Cream | 5 g at bedtime × 3 days | Local irritation |
| 2% Cream | 5 g once | Local irritation |
| Clotrimazole | ||
| Suppositoriesc | 100 mg × 7 days | Local irritation |
| Suppositoriesc | 200 mg × 3 days | Local irritation |
| 1% Cream | 5 g bid × 3 days | Local irritation |
| 1% Cream | 5 g/day × 7 days | Local irritation |
| Miconazole | ||
| Suppositoriesc | 100 mg/day × 7 days | Local irritation |
| 2% Cream | 5 g/day × 7 days | Local irritation |
| 4% Cream | 5 g at bedtime × 3 days | Local irritation |
| Nystatin tabletsc | 100 000 unit tablet × 14 days | Local irritation |
| Tioconazole 6.5% cream | 4.6 g single dose | Local irritation |
| Terconazole | ||
| 0.4% Cream | 5 g/day × 7 days | Local irritation |
| 0.8% Cream | 5 g/day × 3 days | Local irritation |
| Suppository 80 mgc | 1 each day × 3 days | Local irritation |
GI, gastrointestinal.
b Nonprescription alternatives available for most topical drugs with treatment for 3–7 days.
c Presence of vulvar disease requires additional use of cream directly on rash for 3–7 days.
ANTIFUNGAL AGENTS
Amphotericin B is available in four parenteral forms: amphotericin B deoxycholate, liposomal amphotericin B (AmBisome), amphotericin B lipid complex (Abelcet), and amphotericin B colloid dispersion (Amphotec, Amphocil). Adverse effects from the parenteral preparation include fever, chills, electrolyte disturbances, and renal insufficiency. The oral formulation is no longer commercially available.
Ketoconazole is uncommonly used as an oral formulation in the United States because of more frequent incidence of adverse effects, less predictable absorption, higher frequency of drug–drug interactions, and comparatively poor activity against Candida species (all relative to other available azoles), oral ketoconazole is rarely used. It is available as a tablet or cream. Oral absorption is enhanced when the gastric pH is less than 4.0. Achlorhydria has been documented in HIV-infected patients and, when present, may interfere with ketoconazole absorption.48 Itraconazole is a triazole compound available in a cyclodextrin suspension, capsule, or intravenous preparation. The cyclodextrin suspension has enhanced bioavailability compared to the capsule formulation. Absorption is improved when taken after a full meal. Fluconazole was the first triazole compound released in the United States. It is more completely absorbed than itraconazole or ketoconazole because its absorption is not dependent on gastric acidity or food intake. It is available in suspension, tablet, and parenteral forms. Voriconazole and posaconazole are triazoles with expanded in vitro activity against Candida species, including many fluconazole resistant strains (e.g.,C. krusei and selected C. glabrata).
Results from recent clinical trials suggest that the expanded-spectrum triazoles are reasonable alternatives for mucosal candidiasis. Dupont and colleagues presented data on a blinded, randomized study of voriconazole 200 mg twice daily versus fluconazole 200 mg daily for the treatment of EC.49 There was no difference in the number of persons with endoscopically proven cure after 2–6 weeks of therapy; 94.8% of the voriconazole group (n = 115) versus 90.1% of the fluconazole group (n = 141). In one randomized study, posaconazole 100 mg suspension/day compared favorably to fluconazole 100 mg suspension/day for treating OPC in persons with HIV infection.50 The clinical cure rate was 92% (n = 169) for posaconazole versus 93% (n = 160) for fluconazole. Similarly, posaconazole compared favorably to fluconazole in a dose-ranging study for the treatment of oral candidiasis associated with HIV infection.51
In general, the side effects of ketoconazole, itraconazole, fluconazole, voriconazole, and posaconazole are similar. The more commonly reported side effects are headache, dyspepsia, diarrhea, nausea, vomiting, hepatitis, and skin rash.52 Voriconazole can cause reversible photopsia.49 Prolonged administration of azoles may require surveillance of liver enzymes to monitor for hepatotoxicity. There are a number of significant drug interactions with each of these medications (Table 46-2).
Table 46-2 Important Drug Interactions with Selected Antifungal Medications
| Interacting Drug | Antifungal | Effect and Manifestations |
|---|---|---|
| Anticoagulants, oral | F, K, V | Anticoagulant levels (prolonged prothrombin time, bleeding) |
| Antihistamines: H1-blockers (excluding loratadine) | I, F, K | Antihistamine levels (ventricular arrhythmias) |
| Carbamazepine | I | Itraconazole levels (decreased efficacy) |
| Contraceptives, oral | I, F, K, V | Efficacy of oral contraceptives (pregnancy) |
| Cyclosporin/tacrolimus | I, F, K, V | CSA/tacrolimus level (CSA/tacrolimus toxicity) |
| Didanosine | I, K | Complex interaction with levels of involved drugs |
| Digoxin | I | Digoxin levels (digitalis toxicity) |
| HMG-CoA inhibitors | I, F, K | HMG-CoA inhibitor levels (rhabdomyolysis) |
| Oral hypoglycemics | F, K | Oral hypoglycemic effect (hypoglycemia) |
| Isoniazid | I, F, K, V | I, F, K levels (decreased efficacy) |
| H2-blockers/proton pump | I, K, V | I, K levels (decreased efficacy); V levels with omeprazole |
| Phenytoin | I, F, K, V | Complex (I, K, V levels; V, F, phenytoin levels) |
| Rifabutin | F, V | F levels (uveitis); V levels (decreased efficacy) |
| Rifampin | I, F, K, V | I, F, K levels (decreased efficacy) |
| Theophylline | I, F, K | Theophylline levels (toxicity) |
F, fluconazole; I, itraconazole; K, ketoconazole; V, voriconazole; HMG-CoA, 3-hydroxy-3-methylglutaryl coenzyme A; CSA, cyclosporin A.
Caspofungin is effective for oral and EC including fluconazole-refractory disease.53,54 In a randomized double blind study of caspofungin versus amphotericin B for the treatment of candidal esophagitis involving 128 patients, 80% of whom were HIV-infected, caspofungin was at least as effective as amphotericin B (endoscopic cure rates were 74% for caspofungin 50 mg, 89% for caspofungin 70 mg, and 63% for amphotericin B 0.5 mg/kg). Therapy was discontinued due to toxicity in 24% of patients treated with amphotericin B compared to 4% and 7%, respectively, for patients treated with caspofungin.53 Adverse events such as fever, nausea, vomiting, flushing, and infused-vein complications are typically mild.
Micafungin is an echinocandin recently approved for the treatment of EC and prophylaxis for invasive fungal infections among stem cell transplant recipients with neutropenia. Two recently completed54a,54b examining micafungin in the treatment of invasive candidiasis and candidemia should result in its approval for this indication. Concerning the treatment of EC, there are three studies examining the use of micafungin in HIV-infected patients. In the first of these studies, 120 patients with EC confirmed by endoscopy were randomized to receive micafungin at incremental doses ranging from 12.5 to 100 mg daily. Endpoints included clinical and mycologic response at end of therapy and relapse of 2 weeks. The data suggested that micafungin was effective at all doses, but 75–100 mg daily were the most effective doses for healing mucosal lesions.55 A subsequent study comparing micafungin at 50, 100, and 150 mg daily to fluconazole 200 mg daily among 245 patients with HIV-associated EC demonstrated similar observations, specifically, that micafungin at 100 and 150 mg provided efficacy comparable to fluconazole 200 mg daily (micafungin 84%, fluconazole 87%). Notably, 7% of micafungin recipients had a relapse or reinfection during the 2 week follow-up period.56 In the last of these randomized studies, micafungin at 150 mg/day was compared to fluconazole 200 mg/day for a minimum of 14 days with 2–4 week follow-up. Over 500 patients were enrolled into this trial which resulted in a successful outcome in 87% of micafungin and 87% of fluconazole patients.57 Relapses at 4 weeks were seen in 15% compared to 11% of micafungin and fluconazole recipients, respectively. The aggregate data from these trials suggest that micafungin is acceptable therapy for patients with primary EC.
Anidulafungin was recently approved for EC and invasive candidiasis including candidemia. The data supporting the use of anidulafungin in EC is perhaps the most robust among the echinocandins. In the largest study to date, 601 patients with esophagitis were randomized to receive either anidulafungin 50 mg daily or fluconazole 100 mg daily in a phase 3 randomized, double-blind, double-dummy trial.58 Primary treatment duration was two to 3 weeks with either agent. As expected, Candida albicans was the dominant pathogen in this study, accounting for over 95% of infections in both arms of the trial. At the end of therapy, anidulafungin was as effective (97.2%) as fluconazole (98.8%) in the treatment of EC. Clinical and microbiologic response at end of therapy were similar in both arms; however, there was a higher relapse rate in the anidulafungin arm (,30% in a 6 week follow-up period compared to ∼10% with fluconazole). The results of this study raise concerns about the durability of response to echinocandins among patients with EC, and this requires further investigation. Our present understanding suggests that echinocandins are very effective in the primary treatment of EC, but because of concern due to generally higher than expected relapse rates, this should lead clinicians to follow these patients carefully after a successful course of therapy.
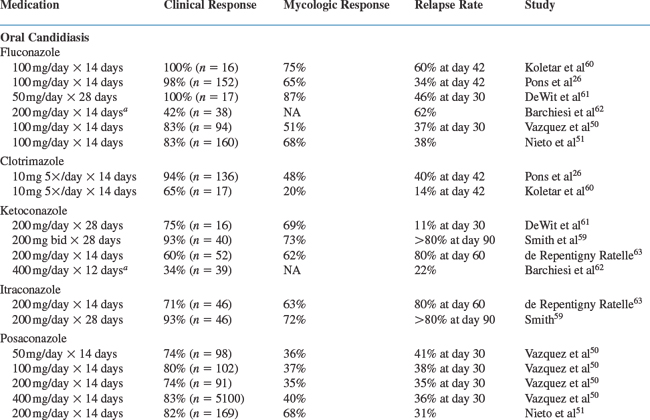
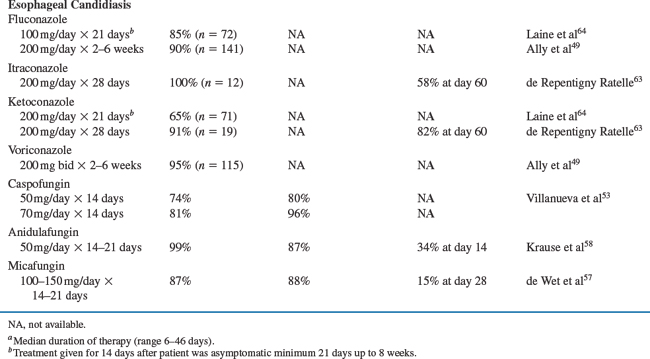
A number of controlled trials of currently approved medications for the treatment of oral and EC are listed in Table 46-3. Response rates range from 34% to 100% in studies of treatment for oral and esophageal disease.59–67 In clinical experience, the response rates to standard antifungal treatments are generally 75% to 95%. There are few significant differences in response to topical versus systemic therapies or among the various systemic therapies for OPC. Thus, it is reasonable to conclude that clotrimazole, ketoconazole, fluconazole, itraconazole, and voriconazole are probably equivalent as acute treatment for most cases of OPC. Mild cases of OPC can be treated with clotrimazole or nystatin initially for 7–14 days. Nonresponsive cases or patients with more severe disease should use fluconazole 100–200 mg/day or itraconazole 200 mg/day for 7–14 days.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree