A husband and wife, aged 76 and 77, respectively, are new patients to a medical practice. The wife mentions that along with having seen multiple direct-to-consumer promotions emphasizing the importance of “healthy living” and the role of early detection in cancer, they recently watched a close friend die of prostate cancer. The wife mentions that she has had “about seven or eight mammograms” in her life, starting when she was 44 years old, but her last test was several years ago, and she is now very worried that she has not been sufficiently proactive about her health. She has come to schedule a mammogram. She would also like to get a prescription for raloxifene, after seeing an advertisement about its bone and breast health benefits in Ladies Home Journal . She states that her husband has “never liked going to the doctor,” and has never previously had a serum prostate-specific antigen (PSA) test, but she has decided, on the basis of their friend’s experience, to “put her foot down.” She also would like to schedule both of them for colonoscopies. Both are now retired; the husband was a construction worker, and the wife, an elementary school teacher. The husband states that except for an incarcerated hernia requiring surgical intervention and a traumatic crush injury to his left shoulder caused by an on-the-job accident, he has no significant medical history. Her medical history is significant for mild hypertension, controlled with the use of a thiazide.
Public health messaging about the power of prevention and early detection has been both pervasive and persuasive. However, given its intuitive, “common sense” appeal, it is also frequently presented in an overly simplistic manner that belies the true complexity of decision making in this field, particularly in the elderly. Benefits may be overstated, and potential harms unrecognized or unconsidered. This chapter is intended to provide a review of the general principles of cancer screening and prevention, as well as a focus on the specific issues unique to older adults; these concepts should facilitate informed, individualized discussions with patients.
First and foremost, it is essential to realize that screening and prevention are fundamentally different activities from treatment of established disease. In the case of treatment, the baseline status of the population is one of symptomatic illness; individuals are actively seeking relief from a specific problem. Screening and prevention, however, deal with a population not overtly affected by the condition of interest and in whom the vast majority will never go on to acquire the disease. It is difficult to make an essentially healthy person better off than he or she already is; as such, the level of acceptable harm due to screening and prevention is lower than for a treatment scenario. The concept of primum non nocere is of particular relevance in the arena of prevention and screening, where the potential for the balance of benefits and harms to tip in the wrong direction rests at a different baseline than with treatment.
Analytic Framework: Rejecting Intuitive Thinking in Screening and Prevention
One of the most efficient tools developed to help clinicians and researchers sort through the salient elements related to the utility of a screening or prevention intervention is the analytic framework. Figure 2-1 depicts sample analytic frameworks (adapted from the U.S. Preventive Services Task Force) for prevention and screening activities, respectively.
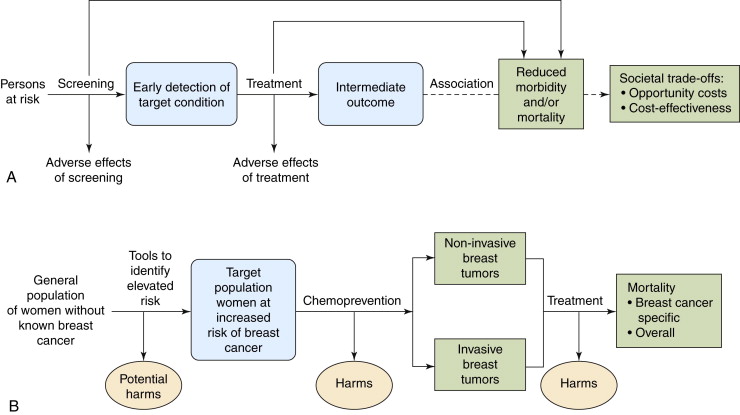
The analytic framework demands that attention be paid to (1) the population under consideration for the intervention (different groups might benefit more or less from a given screening or intervention practice, and proof of efficacy in one group does not automatically equate to utility for all populations); (2) the specifics of the intervention in question; (3) potential harms generated by the application of screening test or preventive agent; (4) potential harms generated by diagnostic follow-up or treatment of a disease; and (5) the precise nature of the potential beneficial outcomes of the intervention. The framework makes a point of explicitly delineating the difference between an intermediate outcome and a true health outcome. This is a useful reminder in screening and prevention efforts because a change in a laboratory value or radiographic examination does not necessarily equate to a decrease in deaths or a clinically meaningful reduction in morbidity for the patient. Although intermediate outcomes are quicker and easier to obtain in studies of screening and prevention interventions because they occur with far greater frequency in an asymptomatic population than “hard” outcomes such as death, it is frequently difficult if not impossible to project with confidence how well they truly predict for endpoints with more clinical impact.
The framework’s careful elucidation of the possible burdens associated with a given screening or prevention behavior is also of great importance: because these practices generally appear essentially innocuous (e.g., a blood draw, an x-ray, or ingestion of a substance already found in other foods) in an asymptomatic population, any associated potential harms are frequently overlooked or discounted. As the framework shows diagrammatically, any benefit of screening or prevention is linked to resulting therapy, so both the benefits and harms of therapy must be considered. Even if an intervention has been demonstrated to reduce disease-specific mortality in some individuals, the practice could still potentially be of net harm to a population, depending on the frequency and severity of associated complications that its use generates.
Finally, the framework is also useful in that it rejects mental shortcuts and a reliance on personal experience, opinion, or assumptions in favor of a series of defined links in a chain of evidence to prove the final net utility of an intervention. This is absolutely critical in the realm of prevention and screening activities because there are strong obfuscating biases operating that can mislead even the most astute clinician, if he or she relies on experience, personal observation, or logical deduction to evaluate the worth of these practices.
Biases in Screening and Prevention Studies
The first of these biases is known as the healthy volunteer effect . This bias occurs because there are fundamental differences between people who are interested in and choose to participate in screening and prevention activities, and those who do not. Persons who participate in early detection or preventive efforts are often more attuned to health messages (e.g., exercise more, smoke less), come from higher educational and socioeconomic strata, are more likely to be compliant with medical advice, and have a generally superior baseline health status, as compared with those who are not interested in such activities. The healthy volunteer effect has been documented in a range of screening and prevention studies: for example, in the Prostate, Lung, Colorectal, and Ovarian (PLCO) Cancer screening trial, investigators found that participants in both the screening and control arms consistently showed lower-than-expected mortality rates (when compared with the general population) for cardiovascular, respiratory, and digestive diseases, diabetes, and all cancers other than those screened for in the study. Even injuries and poisonings occurred about half as frequently as would be expected. An intervention’s apparent success may be entirely attributable to other confounding characteristics that track with the desire to be screened or engage in preventive activities.
A second confounding factor related to screening is known as lead-time bias . Any early detection tool will advance the date of diagnosis forward in time from that of symptomatic presentation. However, it does not automatically follow that a person will live longer as a result of this activity. Figure 2-2 depicts this concept. In this case, it can be seen that although early detection, by definition, shifts the date of diagnosis to an earlier point of time and, as a result, lengthens the period of life during which the person is known to have disease, it has no impact on the time of death. She simply spends more of her life as a cancer patient.
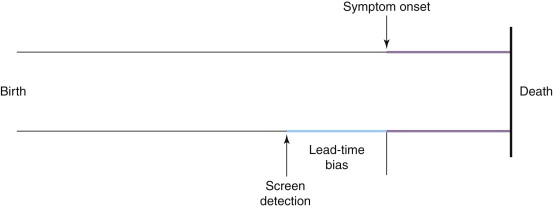
Lead-time bias explains why survival is a particularly misleading endpoint in screening trials, as opposed to disease-specific mortality. To demonstrate this conceptually, take as a hypothetical example a disease that kills 100% of people 4 years after the onset of physical symptoms. The 5-year survival rate is therefore 0%. A new screening test is developed that can diagnose the disease 5 years before symptom onset. The 5-year survival for screen-detected disease therefore rises to 100%, even though nothing has been done in this scenario that will affect the outcome of the disease. Mortality rates are not subject to lead-time bias because they deal with an entirely different denominator: whereas 5-year survival is the number of individuals with the disease alive after 5 years divided by the number of individuals diagnosed with the disease, mortality is the number of individuals who have died from the disease divided by the total population at risk for the disease.
This highlights an important difference between trials of screening and prevention: the usual endpoint used to evaluate efficacy. In the case of cancer screening, as noted previously, the primary endpoint should be cause-specific mortality . However, in prevention trials, the primary endpoint is generally cumulative cancer incidence . The ultimate goal of primary disease prevention is to decrease mortality. Practically speaking, however, few if any cancer prevention trials are large enough or long enough in duration to detect a difference in cancer mortality. In fact, none of the chemoprevention trials that are discussed in this chapter have shown an improvement in cause-specific or overall mortality. In the case of the elderly, a reduction in cancer incidence may never translate into improved cancer mortality because of limited life expectancy. However, the diagnosis of cancer is important in and of itself as a health outcome because it has such a major impact on overall health and because treatments triggered by the diagnosis can be so morbid, particularly in the elderly.
Length-biased sampling is a third form of bias inherent in screening programs. Early detection tools are more effective at identifying slower-growing, less lethal lesions than rapidly progressing ones. This occurs because although every tumor has a given window of time between the threshold of detectability and the appearance of symptoms (the target period of early detection efforts), less aggressive cancers will have a longer preclinical period of growth than more rapidly fatal cancers. As such, a screening tool applied at set intervals has a greater likelihood of detecting these slowly progressive, more favorable lesions than those tumors that quickly advance to a symptomatic state. This does not automatically mean that early detection has had a beneficial impact on the course of the disease; screening programs may simply “stack the deck” with more indolent lesions.
The most extreme form of length-biased sampling is a highly counterintuitive concept termed overdiagnosis . Overdiagnosis occurs when a cancer is detected that would never have gone on to cause problems for the individual. This can occur for two reasons: (1) despite its histological appearance, the lesion is essentially indolent and has no malignant potential or (2) the lesion is so slow growing that the individual would die of another competing cause of death before the cancer would have ever become a health concern. This second mechanism is particularly of concern in older persons; cancer is largely a disease of aging and, even in those who coincidentally have slow-growing cancers, competing causes of death can account for a large proportion of deaths. Overdiagnosed individuals cannot, by definition, benefit from the treatment(s) received, but they are exposed to all of the potential morbidities and even mortality that may accompany the therapy. Table 2-1 provides a summary of these important biases.
| Important biases in cancer screening | Healthy volunteer bias: |
| There are fundamental differences between people who choose to participate in screening and those who do not; persons that participate may tend to be more attuned to health messages, come from higher educational and socioeconomic strata, and have a generally superior baseline health status | |
| Lead-time bias: | |
| The interval between diagnosis at the asymptomatic stage (by screening) and by symptoms; by advancing the date of diagnosis, screening adds apparent survival time compared with symptomatic detection, but this may not translate into a longer life span | |
| Length-biased sampling: | |
| Screening tools disproportionately detect slower-growing, more latent cancers compared with symptomatic detection | |
| Overdiagnosis: | |
| A situation where, despite its pathological appearance, a cancer either has no malignant potential or will not affect remaining life span as the person will die of another cause first | |
| Important considerations for screening and prevention in the older patient | Limited life expectancy and presence of comorbidities: |
| Can increase probability of overdiagnosis and overtreatment, as absolute potential for benefit of screening and prevention decreases with age | |
| Increasing likelihood of harm from preventive agents, treatments: | |
| Older populations may not be as resilient to the toxic effects of chemopreventive agents or the stresses of surgical interventions | |
| Limitations of most screening and prevention efficacy trials in the older population: | |
| Most trials have excluded older patients, meaning that evidence of benefit is extrapolated/assumed to be true in this group |
The potential benefits of screening are a reduction in mortality (overall or disease-specific), or, at minimum, clinically important morbidity associated with the cancer. As effective screening is applied to older populations, because all causes of death become more common with age, it becomes less likely that overall mortality rates will be affected and more probable that only disease-specific mortality will change.
Commonalities between Cancer Screening and Prevention in the Elderly
Some of the core principles in making the personal decision about preventive interventions are similar to those involved in screening decisions. Just as with screening, the target population for cancer prevention is generally healthy; hence, careful consideration must be given to both benefits and harms. The absolute benefits often diminish in the very elderly, whereas the absolute rate of harms may increase. The harms associated with screening and related diagnostic follow-up and treatment often increase with age. For example, advancing age has an adverse effect on postoperative mortality for a range of surgical procedures and associated complication rates. In the case of cancer prevention, strategies frequently involve pharmacologic interventions, which may have unfavorable toxicity profiles in the elderly compared to the young. These considerations may even reverse the benefit-harm balance of screening tests or preventive interventions in the elderly.
Just as with screening, powerful biases can confound the interpretation of prevention studies, leading to overestimation of benefits. “Healthy volunteer” bias is particularly important in prevention studies because adherence to (and interest in) preventive interventions is often associated with underlying robust health and favorable outcomes independent of the actual effect of the intervention. Healthy volunteer bias in clinical screening and prevention trials may therefore make accurate generalization of both benefits and harms to the very elderly difficult.
Unique Aspects in Judging Benefits and Harms of Cancer Prevention in the Elderly
There are also important differences between screening and primary prevention interventions in the elderly. As previously discussed, limited life expectancy may amplify overdiagnosis in screening because even progressive tumors may not grow quickly enough to cause medical problems before the individual dies of competing causes. Delay in time to benefit can also represent an important difference between screening and prevention strategies. “Lead time” before a cancer screening test confers benefit may be on the order of 3 to 15 years. However, the delay in benefits from certain preventive interventions could, in some cases, be far longer if the intervention acts at early stages of carcinogenesis and may be even more likely than screening interventions to fall beyond the remaining life expectancy of an elderly person considering, for example, difficult changes in lifestyle. In contrast, risk for lung cancer begins to drop within a few years after quitting smoking, so tobacco cessation programs are likely to produce benefits even in the elderly.
With some exceptions, such as episodic single cervical cancer or colon cancer screening tests to detect and remove preneoplastic lesions, preventive interventions are usually long term and require prolonged effort. This is particularly true of dietary change and exercise but also applies to the need to take pharmacologic agents for years. These long-term interventions can be especially challenging in a cognitively impaired person or in someone with the physical limitations of advancing age that limit exercise. This stands in contrast to screening interventions, which are repeating but episodic in nature, and although they may cause distress in a cognitively impaired person (who might not understand what is being done), are usually brief, time-limited encounters.
Case Study: Screening Interventions
The Husband: Prostate Cancer Screening
Although this female patient firmly believes in the power of PSA screening to avert prostate cancer death in her husband, experts strongly disagree over the utility of this modality. Despite explosive uptake of this technology in the United States, for many years only observational studies existed to guide practitioners’ judgement, and such studies are particularly prone to the biases previously mentioned. In 2009, the publication of two randomized controlled trials shed new light onto the issue. The first trial was the Prostate, Lung, Colorectal, and Ovarian (PLCO) cancer screening trial. It assigned approximately 77,000 men, aged 55 to 74 years, at 10 U.S. study sites to receive annual PSA testing for 6 years or to usual care. After 7 to 10 years of follow-up, no statistically significant difference in prostate cancer mortality rates was observed, with a trend toward increased death in the screened group (rate ratio 1.13; 95% CI, 0.75-1.7). Between 40% and 50% of participants in the control group did receive PSA screening at least once outside the confines of the trial, which may have had an impact on the observed effect size, although any potential benefit would remain small.
The second trial, the European Randomized Study of Screening for Prostate Cancer (ERSPC), was a multinational study that randomized approximately 162,000 men between ages 50 and 74 years (with a predefined “core” group of 55 to 69 years) to receive PSA testing (at varying intervals, and with digital rectal examination and transrectal ultrasound, depending on screening center, or no screening). There was about a 20% relative reduction in the risk of prostate cancer death in the “core” screening group after a median follow-up of 9 years. Of note, no statistically significant difference in prostate cancer mortality was observed in the overall study population, and, again, there was a trend toward increased mortality in the oldest enrolled subgroup (70 to 74 years) (rate ratio 1.26, 95% CI, 0.80-1.99). The trial also raised considerable concerns about resulting overdiagnosis; it found that 48 cases of prostate cancer needed to be treated to avert one death from the disease.
Neither of these trials provides direct evidence concerning the efficacy of PSA screening for men—such as this patient—who are 75 years and older. Additionally, because most men 75 years and older have a reduced life expectancy, few would be expected to live long enough to experience a mortality benefit from screening. There is also evidence to suggest that any net benefit of treatment with radical prostatectomy for diagnosed prostate cancer may be largely limited to men younger than 65 years.
As stated previously, potential harms must always be weighed against likelihood of benefit when deciding the worth of a clinical intervention. In the case of PSA testing, important possible harms to the individual besides the documented potential for overdiagnosis and overtreatment of latent disease include false-positive results and resulting unnecessary diagnostic procedures (including repeat biopsies). Analysis of the PLCO trial has shown that the cumulative probability for a man to receive at least one false-positive PSA test is 13%, and the probability of undergoing resulting invasive testing is 6%, after four rounds of testing. False-positive tests have been shown to have an impact on men’s mental health. Multiple studies have shown that men with false-positive PSA screening test results are more likely to worry about prostate cancer, have an inaccurately elevated perceived risk for the disease, and have sexual function issues compared with those with normal results. These psychological findings have been documented to persist for at least 1 year after the false-positive test, despite diagnostic resolution of the issue (a normal biopsy).
Finally, potential harms associated with therapy for the disease must also be factored into the overall risk-benefit profile of a screening test because the test can confer no benefit without resulting treatment. In the case of prostate cancer, the harms of treatment can be considerable. A study of quality of life among survivors of localized prostate cancer after treatment with radical prostatectomy, brachytherapy, or external-beam radiotherapy found that at 1 year after treatment, depending on choice of therapy, 54% to 75% could not maintain erections for intercourse, 3% to 14% experienced bowel urgency described as “a moderate or big problem,” and 6% to 16% had urinary incontinence at least once a day. Multiple studies have also shown that the postoperative mortality from radical prostatectomy increases with age; as noted previously, this may occur in the context of absence of potential for benefit from the therapy.
This careful review of the uncertainty of benefits, along with the potential harms of screening and therapy, convinces this patient and his wife that he should forgo PSA screening.
The Wife: Breast Cancer Screening
The wife remains concerned that she has not been getting regular mammograms. There have been a number of randomized controlled trials of mammography performed; however, most of these trials are older (approximately 30 years) (which could reduce the true importance of screening relative to treatment, as new therapies have emerged over time) and have important methodological limitations. Several meta-analyses of these trials have estimated an approximate 15% relative reduction in breast cancer deaths after 10 to 14 years of regular mammography screening in women aged 39 to 74 years. However, age is a critical factor affecting the magnitude of risk reduction. The most recent systematic review performed for the U.S. Preventive Services Task Force found that for women ages 50 to 59, the relative risk was 0.86 (95% CrI [credible interval], 0.75-0.99); for women 60 to 69, 0.68 (95% CrI, 0.54-0.87); and for women 70 to 74, there was a (not statistically significant) trend towards increased breast-cancer mortality with screening (RR, 1.12, 95%; CrI, 0.73-1.72). Importantly, of all of the studies, only the Swedish Two-County trials included women between the ages of 70 and 74 years, and no trial has directly evaluated the efficacy of mammography in women aged 75 years and older.
As with prostate cancer screening, potential harms of mammography screening include the risk of overdiagnosis and overtreatment, adverse effects of treatment, and false-positive results, with resulting psychological effects and unnecessary diagnostic procedures. The potential for radiation-induced breast carcinogenesis has also been cited as a concern, although younger populations (e.g., 40 to 49 years) would be at greatest risk for this outcome. Rates of overdiagnosis associated with the use of screening mammography have been estimated at 10% to 30% of all breast cancers diagnosed. Another way of framing these findings is that for every 2000 women screened regularly for 10 years, 10 women will be treated unnecessarily and 1 death from breast cancer will be averted (the latter after a delay of about 5 to 10 years). Importantly, because overall mortality rates (competing causes of death) rise with increasing age, the probability of overdiagnosis and overtreatment in women 70 years and older is likely higher than for other age groups.
False-positive test results are common with screening mammography. One analysis found that after 10 years of regular screening, nearly 50% of women would have at least one false-positive test, and 20% a resulting biopsy. However, the frequency of false-positive results is thought to decrease with increasing age. Screening also may increase the overall frequency of mastectomies. A pooled analysis of randomized trials found that the relative risk of mastectomy after mammography compared with no screening was 1.35 (95% CI, 1.26-1.44).
Psychological distress associated with false-positive mammography screening has been documented as well. A systematic review of the long-term effects of false-positive mammograms found that, compared with women who had received normal results, women with false-positive test results used mental health care professionals more frequently and had higher levels of anxiety, apprehension, and intrusive thoughts specific to breast cancer. False-negative tests (that is false reassurance that the woman does not have breast cancer) can also be of concern; mammography is estimated to miss 1 breast cancer per 1000 women screened per screening round.
After a careful discussion regarding the unavailability of high-quality evidence about the efficacy of mammography for women in this patient’s age range, along with a review of the important potential associated harms—particularly overdiagnosis and overtreatment—the wife decides that she would like to take some time to further consider the information before deciding on whether to be screened for breast cancer.
The wife is also interested in pursuing colonoscopy screening for colorectal cancer for both herself and her husband. She notes that neither has previously received a colonoscopy, although her gynecologist had occasionally performed an in-office guaiac smear; the results of these have always been negative. Her physician points out to her that in-office guaiac smears are not considered an acceptable form of colorectal cancer screening, having never been tested in prospective studies.
Husband and Wife: Colorectal Cancer Screening
Until recently, only the home based fecal occult blood test (FOBT) had randomized, controlled evidence available to demonstrate reductions in colorectal cancer deaths. Several trials of FOBT have consistently shown relative reductions in colorectal cancer mortality of between 15% and 33%, depending on whether the test was administered annually or biennially; this translates into an absolute risk reduction of about one to five deaths per 1000 participants. Of note, most trials only included individuals up to 74 years of age; a single study provides evidence for up to 80 years. Newer fecal immunochemical tests have demonstrated improved sensitivity and specificity compared with guaiac-based tests and have been recommended for use by the U.S. Preventive Services Task Force ( Table 2-2 ).
| Intervention | Modality | Recommendation |
|---|---|---|
| Prostate cancer screening | PSA | Men, <75 years: the current evidence is insufficient to assess the balance of benefits and harms (“I”) Men, 75+ years: Recommends against screening (“D”) |
| Breast cancer screening | Mammography | Women, 50-74 years: Recommends biennial screening (“B”) Women, 75+ years: The current evidence is insufficient to assess the balance of benefits and harms (“I”) |
| Colorectal cancer screening | Fecal occult blood testing, annually Flexible sigmoidoscopy, every 5 years Colonoscopy, every 10 years | Men and women, 50-75 years: Recommends screening (“A”) Men and women, 76-85 years: Recommends against routine screening; there may be considerations that support screening in an individual patient (“C”) Men and women, 86+ years: Recommends against screening (“D”) |
| CT colonography Fecal DNA testing | The current evidence is insufficient to assess the balance of benefits and harms (“I”) | |
| Breast cancer chemoprevention | Tamoxifen Raloxifene | Women, any age, low to average risk for breast cancer: Recommends against routine use (“D”) Women, any age, high risk: Recommends clinicians discuss chemoprevention (“B”) |
| Colorectal cancer prevention | Aspirin/NSAIDs | Men and women, all ages: Recommends against routine use (“D”) |
| Cancer chemoprevention, general | Vitamins A,C, E Multivitamins with folic acid Antioxidants | Men and women, any age: The evidence is insufficient to recommend for or against use (“I”) |
∗ For more detailed information regarding these recommendations, go to: http://www.ahrq.gov/clinic/uspstf/uspstopics.htm
Flexible sigmoidoscopy (which can evaluate the left side of the colon up to the splenic flexure) is another screening option for the couple to consider. A recently published randomized, controlled trial of one-time flexible sigmoidoscopy versus usual care in 170,000 men and women ages 55 to 64 years demonstrated a statistically significant 30% relative reduction in colorectal cancer mortality. Although colonoscopy has the least evidence available to directly demonstrate its efficacy in reducing colorectal cancer mortality, because the procedure is integral to diagnostic follow-up and polyp removal for the other screening options (and, as such, is a necessary step in colorectal cancer screening programs), this has been thought to represent sufficient indirect evidence of efficacy to support its use as a stand-alone screening option. Colonoscopy generally allows for visualization of the entire colon (to the cecum). On the other hand, two recent epidemiologic studies have suggested that the benefits of colonoscopy may be restricted to the left side of the colon. Other screening options under development include computed tomography (CT) colonography and fecal DNA testing; however, evidence regarding the effectiveness of these modalities is still being acquired.
Harms associated with screening vary by the modality used. FOBT in and of itself appears to have the lowest risk of associated adverse events, although its associated false-positive rate (2% to 10%, depending on whether rehydration is used) is of concern because each positive test leads to further evaluation with colonoscopy, which has higher rates of complications. In the most recent systematic evidence review performed in support of the U.S. Preventive Services Task Force, flexible sigmoidoscopy was found to have a rate of serious complications of about 3.4 per 10,000 procedures (including perforation, major bleeding, diverticulitis, and cardiovascular events requiring hospitalization, as well as death). Colonoscopy appeared to have the highest rate of associated serious complications, at 25 per 10,000 procedures. Perforations alone accounted for about 4 per 10,000 procedures.
Although the relative frequencies of harm by age have not been well studied, at least two trials have shown increased risks of perforation with colonoscopy in older adults (older than 60 years). A modeling study performed by two groups from the Cancer Intervention and Surveillance Modeling Network (CISNET) found that although colorectal adenoma incidence does increase with advancing age, for individuals between the ages of 75 and 85, any gains in life-years acquired through screening were small in comparison to the risks of associated complications. Furthermore, as was true for prostate and breast cancer, the increasing frequency of important comorbidities and competing causes of death in this population reduces the likelihood that any benefits of screening (which may take up to a decade or more to appear) will be actualized.
After reviewing the limitations of the evidence for persons aged 75 and older, and after careful discussion of the variable risks associated with each of the colorectal cancer screening strategies, the husband decides he is not interested in pursuing any type of screening. The wife decides that she is uncomfortable with pursuing colonoscopy as a primary screening test, given the review of potential harms, but, as she feels she is in essentially good health, she is interested in at-home FOBT testing.
Case Study: Prevention Interventions
The Husband: Prostate Cancer Prevention
The husband has chosen not to receive prostate cancer screening. However, his friend informed him that a drug that is used to treat benign prostatic hyperplasia (BPH) and baldness has been shown to decrease the risk of developing prostate cancer and that the side effects are relatively mild. This appeals to him, and he wants to know whether he should take it for cancer prevention.
Although not approved by the Food and Drug Administration (FDA) for prostate cancer prevention, a large randomized placebo controlled trial of the 5-alpha reductase inhibitor finasteride (the Prostate Cancer Prevention Trial [PCPT]) does provide good evidence that finasteride at a dose of 5 mg orally per day decreases the risk of prostate cancer. In the trial, 18,882 men aged 55 and older were randomly assigned to take finasteride or placebo for up to 7 years. Over the 7-year period, the rates of prostate cancer diagnosis were 18% and 24% in the finasteride and placebo arms, respectively, for a relative reduction of 25%. Because the study design mandated an end-of-study prostate biopsy in all men who had not previously been biopsied, the high rates of cancer in each study were due to both clinically relevant cancers and those that would not have been detected had it not been for per-protocol biopsy. A subsequent systematic review of the use of 5-alpha reductase inhibitors for prostate cancer prevention estimated the number needed to treat (NNT) to prevent one diagnosis of prostate cancer after about 7 years of finasteride use was about 71.
Side effects of finasteride were modest and included a decrease in volume of ejaculate, a small decrease in libido, and slight increases in erectile dysfunction and gynecomastia. The effects on sexual function were generally reversible. On the plus side, problems associated with urinary obstruction (including urinary urgency, frequency, and retention) were lower in the finasteride arm compared with placebo.
However, the initial report of the PCPT showed a potentially worrisome increase in diagnoses of high-grade (Gleason score 7-10) tumors associated with finasteride (6% compared with 5%). Even though the number of deaths from prostate cancer was the same in each arm, the fear was that the increase in high-grade tumors might ultimately translate into a higher risk of death from prostate cancer. Subsequent analyses have provided evidence that the increase in high grade tumors in men taking finasteride is likely to be spurious because finasteride decreases the size of the prostate gland, leading to an increase in sensitivity of PSA in the detection of high grade tumors. As part of the study design, all men were being routinely screened annually with PSA and digital rectal examinations.
The routine screening of all men in the PCPT brings up a key issue in counseling this patient. Because of the study design, the impact of finasteride on prostate cancer risk is only known in men who are being regularly screened for prostate cancer. PSA testing is known to increase the risk of being diagnosed with prostate cancer by about 100%. Many of these screen-detected cancers are indolent and would never have come to attention had it not been for screening. Therefore finasteride does not bring the risk of being diagnosed with prostate cancer down to the level of risk in a man who is not being screened at all. It is also not known how effective finasteride is in preventing cancers not detected by screening. Because this man declined prostate cancer screening, finasteride may be of little or no benefit.
Given this caveat, he asks whether a specific diet, dietary supplements, or vitamins are known to prevent prostate cancer. Unfortunately, there are no known dietary interventions known to decrease prostate cancer risk. In a randomized trial, selenium and vitamin E did not decrease prostate cancer risk. Evidence regarding most other nutrients and supplements is inconsistent, and there are no randomized trials to inform decisions.
The wife has heard about the use of the selective estrogen receptor modulators (SERMs) tamoxifen and raloxifene to lower breast cancer risk and would like to know if she should take one. As in the case of counseling on prostate cancer chemoprevention, treatment decisions are complex and must be individualized. The risk-benefit ratio changes with age and also depends on the underlying absolute risk for breast cancer. As in the case of prostate cancer chemoprevention, there is evidence from randomized controlled trials to help guide the decision.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree



