Within 1 year of the discovery of x-rays by Wilhelm Roentgen in 1895, radiation was used for the treatment of malignancy. Today, approximately 50% to 60% of cancer patients receive radiation therapy (RT) as part of their disease management. The role of RT is particularly important for the geriatric population, given the association of aging with an increased incidence of cancer, as well as the often present comorbidities in the elderly that may preclude the delivery of more invasive or aggressive treatment alternatives. Radiation therapy is an important treatment option as monotherapy or in combination with other treatment modalities for older patients with cancer.
This chapter will provide an overview of the basic mechanisms and rationale for the use of RT and discuss the process of care and toxicities associated with RT in the management of elderly patients with cancer. Radiation therapy alone is generally well tolerated in the aged, while concurrent chemoradiotherapy (CRT) requires considered patient selection due to increased treatment-related morbidity. External beam radiation delivered by linear accelerators is the treatment delivery method most often utilized by radiation oncologists for treatment of the elderly; however, other RT techniques such as brachytherapy and radiopharmaceuticals may also be useful. The increased precision of modern RT technology, which allows for significant increase in normal tissue sparing, will be discussed because of its potential import in tailoring treatment to the special needs of the aged. Radiation therapy, used in combination with other treatment modalities or as monotherapy, offers a powerful therapeutic tool for the management of the elderly patient with cancer, for both curative and palliative clinical circumstances.
Mechanisms, Rationale, and Process of Care for Radiation Therapy
Radiation oncology deals with the therapeutic application of ionizing radiation to treat benign and malignant diseases. The most common approach used to deliver ionizing radiation is external beam radiation therapy (EBRT), which utilizes high-energy photons, or electrons produced by linear accelerators. Protons and other heavy particles, including neutrons and carbon ions, are less commonly used and continue to be studied. Radioactive isotopes generating beta particles and gamma rays are delivered by brachytherapy, the surgical implantation of radioactive sources into the body to treat cancer, and with systemic radiopharmaceutical treatments.
The benefit of radiation therapy stems from the biological fact that ionizing radiation directly and indirectly damages the genetic material of the cell, the DNA, which controls cell growth and replication. Although normal cells are also in the path of the radiation beam, they have superior DNA repair mechanisms and therefore can more readily repair damage sustained from irradiation. Cancer cells are more susceptible to this DNA damage-related disruption of cell replication and undergo cell death through necrosis or apoptosis. Laboratory studies examining the relationship of age and tumor radiosensitivity in vitro and within animal models are limited. However, the relationship of age and radiation-induced normal tissue toxicity has been more extensively studied. From these studies, it is thought that the mechanism by which radiation affects normal tissue cells is similar in younger and older patients.
When the cancer patient is evaluated for RT, the radiation oncologist determines whether radiation treatment is indicated in the particular clinical circumstance, establishes the specific intent of the treatment, and defines an overall treatment plan. Radiation therapy may be used alone or in combination with surgery or systemic therapies such as chemotherapy. In almost all cases, the aim of RT is to provide local control of a tumor for either a curative or palliative outcome. In the curative circumstance, a patient may accept a greater risk of toxicity associated with higher doses of RT or the addition of concurrent chemotherapy with or without surgery. In contrast, the goal of radiation therapy in the palliative setting is to ameliorate or prevent cancer-related symptoms without causing additional significant morbidity.
The decision to recommend RT and the aggressiveness of its application for elderly patients must be individualized to the clinical circumstance, the patient’s overall functional status, and his or her general medical condition. Clinical experience and the medical literature have concluded that age alone should not preclude the use of RT. The clinical discussion to follow will illustrate that the toxicities experienced by elderly patients receiving RT are not significantly different from or more severe than those of the general cancer patient population. However, other issues beyond age may be relevant to the consideration of radiation therapy in the elderly patient. For example, the patient’s general medical condition, functional status, issues of quality versus quantity of life, logistical and social obstacles to treatment, comorbidities, polypharmacy, and neurocognitive status all must be factored into the treatment decision-making process. Tools such as the Comprehensive Geriatric Assessment (CGA) allow for a broad appraisal of the physical, mental, social, and functional capabilities and limitations of elderly adults. Such formal evaluation tools may enhance the medical decision-making process regarding the appropriateness of specific treatments, including RT, and also better inform inclusion criteria for clinical trials where older patients had traditionally been excluded solely on the basis of age.
After the radiation oncology consultation, if radiation therapy is deemed indicated and appropriate, the patient undergoes a radiation treatment planning session called a simulation. During the simulation, the patient is positioned on a simulated treatment couch in the exact position that will be used during actual daily treatment on the linear accelerator ( Figure 7-1 ). Immobilization devices, such as a custom face mask for head immobilization or body molds or casts, are often used to help provide stable and reproducible patient positioning to enhance the accuracy of the daily treatment. Patients who are able to cooperate with the simulation and the daily treatment setup are more likely to receive accurate and precise targeting of RT throughout their treatment course. However, patients with cognitive impairment, dementia, severe anxiety, or other functional deficits that may limit compliance offer challenges to the treatment management. Such circumstances may require modifications such as alteration of treatment field size, use of anti-anxiety medications, prescribing a shortened course of therapy, or even, rarely, anesthesia.
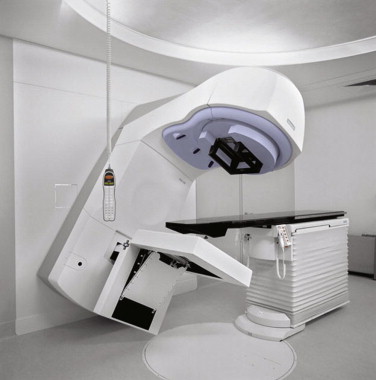
During the simulation, imaging with x-rays, fluoroscopy, or computerized tomography (CT), sometimes combined with positron emission tomography (PET) or magnetic resonance imaging (MRI) provide visualization of the region to be treated with radiation. The images obtained are electronically transferred to a specialized dedicated treatment-planning computer, where the tumor target is defined and surrounding normal structures are contoured by the radiation oncologist. The radiation oncologist then works with a team of physicists and dosimetrists to select the appropriate radiation dose, beam energy, and beam direction(s) required to effectively treat the tumor while limiting the dose to normal tissues and critical structures. Treatment planning can take anywhere from several hours to several days depending on the complexity of the case.
A course of RT is typically fractionated, meaning the total dose is delivered in smaller divided doses over time, typically over several days or weeks. Conventional curative courses of RT utilize a daily dose of 180 and 200 cGy given 5 days per week and lasting between 4 and 9 weeks in duration. Palliative courses of radiation are often shorter, ranging from a single treatment to 20 treatments over 4 weeks’ time. Fractionation exploits a number of radiobiological principles that increase the therapeutic benefit of radiation therapy. Fractionation allows for normal tissues to repair sublethal DNA damage between fractions, which enhances the patient’s tolerance of the treatment. In addition, fractionation allows for tumor cells to undergo redistribution into more radiation-sensitive phases of the cell cycle between fractions and for reoxygenation of tumor cells between fractions, making them more sensitive to RT.
A change in fractionation scheme alters the biological effect of radiation therapy. In some cases, such as hyperfractionation (two treatments per day separated by a minimum of 4 hours), the goal is intensification of dose to improve tumor control within the limits of acute and late toxicities. By achieving a higher total dose to the tumor within the same or shorter time period without a substantial increase in acute or late toxicity, the probability of tumor control can be enhanced. Selected elderly patients with head and neck cancer have been shown to tolerate variations of such aggressive regimens.
In other instances, modifications in fractionation are made to accommodate patients with mobility or transportation issues while still providing effective tumor response, particularly in the palliative setting. An extended duration of RT can be problematic for some older patients with mobility issues, certain comorbidities, or other logistical issues hindering daily transport for treatment. In this regard, shortened treatment courses, called hypofractionated , made possible by advanced treatment planning methods, can enhance the applicability of RT to the unique needs of the elderly cancer patient. Hypofractionation without compromising tumor control requires an increase in the size of each fraction, and therefore will result in increased late normal tissue toxicity. However, late toxicity may not be of concern for the older patient who stands to benefit from the symptomatic relief and shortened treatment time offered by hypofractionated RT.
A review of the literature on short-course RT for aged cancer patients by Donato, et al. describes various regimens used for malignancies of the brain, breast, lung, prostate, bladder, and rectum, demonstrating safe and effective palliation of symptoms in each organ system. One exception is hypofractionated palliative RT for head and neck cancers, in which the clinical benefits do not appear to outweigh toxicities. A special case of hypofractionation, called stereotactic body radiation therapy (SBRT), can be used with curative intent for a number of tumor sites and is discussed in the section on Cutting Edge RT Techniques.
Balancing Toxicity of Radiation Therapy with Therapeutic Goals
The goal of radiation therapy is to maximize the probability of tumor kill while minimizing the risk of normal tissue injury. This risk-benefit analysis is influenced by the intent of treatment. For curative treatment, higher radiation doses are required to maximize tumor control. Such higher doses may be associated with increased normal tissue toxicity. For palliative cases, the aim is to deliver the minimum dose that is able to achieve durable improvement of the tumor-associated symptom while also minimizing the risks of RT-induced toxicity.
With the exception of the RT-associated symptom of treatment-induced generalized fatigue—which is not universal and most of the time does not occur—radiation toxicities and their associated side effects are local and site-specific. RT-related side effects can be categorized as acute (those symptoms occurring during the treatment course), subacute (those symptoms occurring within 3 months of treatment), and late toxicities (those occurring beyond 3 months of completion of RT).
Acute and Subacute Effects of Radiation Therapy
Acute toxicity occurs in normal epithelial tissues or other rapidly dividing cell populations within the treatment field. It demonstrates the equilibrium between cell death and stem cell proliferation in response to radiation damage. Clinically and histopathologically, the acute reaction is also characterized by inflammatory and immune responses to both radiation-induced tumor cell death and damage to normal tissue. Acute side effects are expected to occur to some degree in most curative courses of RT. Depending on the site of treatment, toxicities may include hair loss, dysphagia, odynophagia, skin erythema or desquamation, nausea, vomiting, oral mucositis, esophagitis, pneumonitis, enteritis, proctitis, and cystitis. Acute toxicities, if they are to occur, typically happen approximately 2 to 3 weeks after initiation of daily radiation therapy ( Table 7-1 ), and are only infrequently of significant severity to warrant brief breaks in treatment or the discontinuation of therapy. The vast majority of acute side effects are managed by outpatient pharmacological interventions or nutritional modifications, are usually self-limited, and resolve within several weeks of completing the course of radiation treatment.
| Reaction | Management |
|---|---|
| Skin erythema/desquamation | Aloe vera; hydrocortisone (0.5%, 1%) cream; silver sulfadiazine cream |
| Mucositis | Sodium bicarbonate oral gargle; diphenhydramine/viscous lidocaine/aluminum hydroxide mix; oral sucralfate suspension; amifostine |
| Odynophagia/dysphagia | Hydrocodone/acetaminophen elixir, oral sucralfate suspension, nystatin suspension; preventative swallow exercises |
| Pneumonitis | NSAIDs, oral steroids |
| Nausea/vomiting | Prochlorperazine; ondansetron |
| Diarrhea | Loperamide; diphenoxylate/atropine |
| Cystitis/dysuria | Phenazopyridine; oxybutynin; tolterodine |
| Proctitis | Hydrocortisone (1%, 2.5%, 10%) ointment |
| Myelosuppression | Transfusion; brief treatment break |
| Fatigue | Exercise; psychosocial intervention; supportive care |
Acute Effects and the Suitability of Radiation Therapy in Treatment of the Elderly
The notion that elderly patients should be offered noncurative regimens or not offered radiation as a treatment option at all because they may not be able to tolerate a curative course of radiation therapy is not supported by clinical experience or the peer-reviewed literature. Indeed, aging is associated with changes in molecular and biochemical pathways at the cellular level. However, experiments have been performed on mouse and pig skin, mouse lip mucosa, and vascular smooth muscles cells in vitro , all of which describe similar acute normal tissue radiosensitivity across varying host ages. One study on the acute radiation response in skin of young and old rats reported a decrease in tissue radiosensitivity correlated with age. Clinically, many retrospective studies support the view that RT alone does not cause significant differences in toxicity between younger patients and older patients without other severe comorbidities and reasonable performance status. Zachariah et al. retrospectively examined the records of 203 patients aged 80 or older who received RT at facilities associated with Moffitt Cancer Center over a 7-year period and found that more than 90% were able to complete treatment without significant complications. This completion rate is similar to the overall population of patients treated with RT. A similar study by Wasil et al. also concluded that older patients safely tolerate radiation therapy both for curative and palliative intent, with more than 80% of patients able to complete their planned treatment course. Even CRT can be offered to provide improved outcomes in the elderly population for such diseases as locally advanced head and neck cancer, lung cancer, and esophageal cancer. Such aggressive regimens do result in an increased acute side effect profile in all age groups. Elderly patients may be more vulnerable to such stresses; thus careful patient selection and aggressive supportive management may be required.
During the course of radiation therapy, patients are scheduled to see the radiation oncologist a minimum of once weekly for assessment of acute toxicities, but can and should be seen more often depending on the needs of the patient. Most common side effects are easily managed with over-the-counter medications and skin care products, though some side effects may require prescription-strength medications, and at times more aggressive interventions (see Table 7-1 ). All cancer patients benefit from the multidisciplinary management by social workers, dieticians, transportation aides, and other support staff. This is particularly true for many geriatric patients who battle their disease with the added burdens of social isolation, a weakened support structure, self-denial of symptom severity, and decreased patient concern regarding the critical nature of self-care and personal advocacy. Straightforward side effects may be rationalized, ignored, and exacerbated by patient ennui resulting in an increased probability of more severe treatment-related sequelae such as dehydration with electrolyte imbalance and/or dysphagia leading to malnutrition and cachexia. Although the results of a study reviewing 210 patients older than 74 years treated with a variety of aggressive RT regimens for varying sites of disease concluded that curative RT is well-tolerated in older patients, the authors, for reasons similar to those mentioned earlier, also recommended more vigilant management of mucositis and diarrhea in elderly patients, who are prone to dehydration.
Not uncommon in the geriatric population is the use of pacemakers and implantable defibrillator devices. There is a rare possibility of radiation-induced malfunction of these devices when they are directly in or near the treatment beam. Caution should be taken by the radiation oncologist by consulting with the patient’s cardiologist and a medical physicist to ensure that the treatment will not cause untoward effects on the function of these devices.
Subacute Effects
The most common subacute side effect of RT is radiation pneumonitis, in patients whose normal lung is necessarily within the treatment field as required in the treatment of lung cancer or breast cancer. This side effect occurs in the days and weeks following treatment and is characterized by mild symptoms of breathlessness and a dry cough. It is usually managed conservatively. In patients taking long-term steroid medication for preexisting medical problems or in those patients with severe lung disease such as chronic obstructive pulmonary disease (COPD), radiation pneumonitis may be much more severe and require management by a pulmonary specialist to prevent a more serious progression of the symptoms.
Late Effects
Late effects developing in patients who have received radiation therapy are usually associated with damage to vascular, lymphatic, nervous, and/or connective tissues or other cell populations with a low mitotic rate. These effects can occur anytime from 3 months to many years after radiation exposure. Most such problems occur between 9 and 24 months after completion of treatment; they rarely occur beyond 5 years. Most late effects caused by radiation do not rise to a level that meaningfully affects the patient’s quality of life. Typically, signs and symptoms such as chronic skin changes of epidermal telangiectasia and tanning, subdermal fibrosis, and mild-to-moderate soft-tissue fibrosis comprise the majority of radiation-induced late side effects. These side effects tend not to cause significant morbidity for the patient. As with acute reactions, late toxicity must be localized to the treatment field and is dependent on total dose, fractionation, and volume of the critical organs irradiated, and rare idiosyncratic patient response to radiation. In contrast to acute effects, most late-effect damage is irreversible. However, the use of tocopherol (vitamin E) and pentoxifylline has been reported to improve late-effect changes of soft tissue fibrosis in symptomatic patients. Hyperbaric oxygen therapy has also been shown to relieve several radiation-induced late side effects. Infrequently, significant permanent decrement in the patient’s quality of life can result. For example myelopathy, cataracts, xerostomia, gastrointestinal stricture, pulmonary fibrosis, lymphedema, nephropathy, osteoradionecrosis. and soft tissue scarring and/or necrosis are possible rare late outcomes, even in properly administered radiation therapy.
Laboratory data do not suggest that worse late toxicities of RT are related to host age. For example, several in vitro studies on fibroblasts, which are thought to be the principle cells involved in late radiation response, did not demonstrate a relationship between radiosensitivity and age. Animal studies of individual organ systems do not correlate aging with more severe late reactions, and several studies even suggest older animals show a greater resistance to the late effects of radiation. In Ruifrok et al., the latency period between spinal cord irradiation and the development of myelopathy was significantly longer in older versus younger rats. Another pair of experiments from separate laboratories, examining radiation-induced nephropathy, both demonstrated decreased renal radiosensitivity in older pigs and rats. In one clinical circumstance, the findings are less clear-cut. For CNS malignancies, some reports suggest elderly patients receiving brain irradiation to large treatment volumes are at greater risk of cognitive decline as a result of therapy. However, in this case, vascular comorbidities such as hypertension, diabetes, and atherosclerosis, with a higher incidence in the elderly, confound the causal analysis between age and toxicity. In addition, the conventional wisdom regarding cognitive changes associated with cranial irradiation has recently been augmented by the understanding that patients with CNS primary and metastatic disease often suffer preradiation neurocognitive problems. When baseline neurocognitive measures are made before RT, the imputed effects of RT fall away. Nevertheless, due care to limit the amount of brain irradiation in young and older patients remains a current tenet of good radiation oncology practice.
The concern over late toxicity may also be less relevant for some elderly patients with shorter life expectancy. In general, the risk of late complications can be reduced by decreasing the per-fraction dose. However, in such a case, the total course of radiation must be extended in order to achieve a high enough dose to control the tumor. For some patients, improving present quality of life is the higher priority over minimizing the possibility of late effects. In such cases, which are usually palliative, a shortened or hypofractionated course of RT is often effective and may provide the benefit of both symptom relief and abridged treatment days.
Comorbidities and Radiation Therapy in the Elderly
As previously described, clinical and laboratory studies do not suggest that aging alone affects the mechanisms of acute or late radiation response. However, aging is associated with comorbid illnesses, as well as with a decline in physiologic reserve. It is likely that these factors play the most relevant role in the selection of elderly patients for RT as well as their tolerance of it. Common medical conditions faced by the elderly such as hypertension, atherosclerosis, heart disease, and COPD are rarely, on their own, contraindications to RT. Rather, treatment and management decisions are influenced by a combination of factors including the anatomical region being irradiated, the volume of critical organs or structures in the treatment field, and the specific comorbidities and associated functional status of the patient. For example, because the older patient can be at increased risk for upper respiratory tract or urinary tract infections, special attention for the development of acute side effects in these organ systems may be warranted during their RT course. In another case, a patient with Parkinsonian tremor may pose a challenge because of his or her difficulty remaining still; however, appropriate immobilization and treatment field design usually obviates significant difficulties with delivery of RT in such patients. Finally, as elderly cancer patients already demonstrate high rates of fatigue and depression, minimizing treatment-related fatigue is particularly important for such patients. Studies suggest the RT-induced fatigue is less severe and lasting than its chemotherapy or combined modality counterparts, and with modern RT techniques further shrinking the irradiated volume and course of therapy, even greater gains have been observed. These examples highlight the heterogeneous composition of the elderly population, who despite comorbidities, with proper individualized assessment and treatment design, are still good candidates for RT. Considering the three major modalities of oncologic care, RT is often a reasonable option for the geriatric patient who may be unable to tolerate the physiologic stresses of surgery or chemotherapy.
Acute and Subacute Effects of Radiation Therapy
Acute toxicity occurs in normal epithelial tissues or other rapidly dividing cell populations within the treatment field. It demonstrates the equilibrium between cell death and stem cell proliferation in response to radiation damage. Clinically and histopathologically, the acute reaction is also characterized by inflammatory and immune responses to both radiation-induced tumor cell death and damage to normal tissue. Acute side effects are expected to occur to some degree in most curative courses of RT. Depending on the site of treatment, toxicities may include hair loss, dysphagia, odynophagia, skin erythema or desquamation, nausea, vomiting, oral mucositis, esophagitis, pneumonitis, enteritis, proctitis, and cystitis. Acute toxicities, if they are to occur, typically happen approximately 2 to 3 weeks after initiation of daily radiation therapy ( Table 7-1 ), and are only infrequently of significant severity to warrant brief breaks in treatment or the discontinuation of therapy. The vast majority of acute side effects are managed by outpatient pharmacological interventions or nutritional modifications, are usually self-limited, and resolve within several weeks of completing the course of radiation treatment.
| Reaction | Management |
|---|---|
| Skin erythema/desquamation | Aloe vera; hydrocortisone (0.5%, 1%) cream; silver sulfadiazine cream |
| Mucositis | Sodium bicarbonate oral gargle; diphenhydramine/viscous lidocaine/aluminum hydroxide mix; oral sucralfate suspension; amifostine |
| Odynophagia/dysphagia | Hydrocodone/acetaminophen elixir, oral sucralfate suspension, nystatin suspension; preventative swallow exercises |
| Pneumonitis | NSAIDs, oral steroids |
| Nausea/vomiting | Prochlorperazine; ondansetron |
| Diarrhea | Loperamide; diphenoxylate/atropine |
| Cystitis/dysuria | Phenazopyridine; oxybutynin; tolterodine |
| Proctitis | Hydrocortisone (1%, 2.5%, 10%) ointment |
| Myelosuppression | Transfusion; brief treatment break |
| Fatigue | Exercise; psychosocial intervention; supportive care |
Acute Effects and the Suitability of Radiation Therapy in Treatment of the Elderly
The notion that elderly patients should be offered noncurative regimens or not offered radiation as a treatment option at all because they may not be able to tolerate a curative course of radiation therapy is not supported by clinical experience or the peer-reviewed literature. Indeed, aging is associated with changes in molecular and biochemical pathways at the cellular level. However, experiments have been performed on mouse and pig skin, mouse lip mucosa, and vascular smooth muscles cells in vitro , all of which describe similar acute normal tissue radiosensitivity across varying host ages. One study on the acute radiation response in skin of young and old rats reported a decrease in tissue radiosensitivity correlated with age. Clinically, many retrospective studies support the view that RT alone does not cause significant differences in toxicity between younger patients and older patients without other severe comorbidities and reasonable performance status. Zachariah et al. retrospectively examined the records of 203 patients aged 80 or older who received RT at facilities associated with Moffitt Cancer Center over a 7-year period and found that more than 90% were able to complete treatment without significant complications. This completion rate is similar to the overall population of patients treated with RT. A similar study by Wasil et al. also concluded that older patients safely tolerate radiation therapy both for curative and palliative intent, with more than 80% of patients able to complete their planned treatment course. Even CRT can be offered to provide improved outcomes in the elderly population for such diseases as locally advanced head and neck cancer, lung cancer, and esophageal cancer. Such aggressive regimens do result in an increased acute side effect profile in all age groups. Elderly patients may be more vulnerable to such stresses; thus careful patient selection and aggressive supportive management may be required.
During the course of radiation therapy, patients are scheduled to see the radiation oncologist a minimum of once weekly for assessment of acute toxicities, but can and should be seen more often depending on the needs of the patient. Most common side effects are easily managed with over-the-counter medications and skin care products, though some side effects may require prescription-strength medications, and at times more aggressive interventions (see Table 7-1 ). All cancer patients benefit from the multidisciplinary management by social workers, dieticians, transportation aides, and other support staff. This is particularly true for many geriatric patients who battle their disease with the added burdens of social isolation, a weakened support structure, self-denial of symptom severity, and decreased patient concern regarding the critical nature of self-care and personal advocacy. Straightforward side effects may be rationalized, ignored, and exacerbated by patient ennui resulting in an increased probability of more severe treatment-related sequelae such as dehydration with electrolyte imbalance and/or dysphagia leading to malnutrition and cachexia. Although the results of a study reviewing 210 patients older than 74 years treated with a variety of aggressive RT regimens for varying sites of disease concluded that curative RT is well-tolerated in older patients, the authors, for reasons similar to those mentioned earlier, also recommended more vigilant management of mucositis and diarrhea in elderly patients, who are prone to dehydration.
Not uncommon in the geriatric population is the use of pacemakers and implantable defibrillator devices. There is a rare possibility of radiation-induced malfunction of these devices when they are directly in or near the treatment beam. Caution should be taken by the radiation oncologist by consulting with the patient’s cardiologist and a medical physicist to ensure that the treatment will not cause untoward effects on the function of these devices.
Subacute Effects
The most common subacute side effect of RT is radiation pneumonitis, in patients whose normal lung is necessarily within the treatment field as required in the treatment of lung cancer or breast cancer. This side effect occurs in the days and weeks following treatment and is characterized by mild symptoms of breathlessness and a dry cough. It is usually managed conservatively. In patients taking long-term steroid medication for preexisting medical problems or in those patients with severe lung disease such as chronic obstructive pulmonary disease (COPD), radiation pneumonitis may be much more severe and require management by a pulmonary specialist to prevent a more serious progression of the symptoms.
Late Effects
Late effects developing in patients who have received radiation therapy are usually associated with damage to vascular, lymphatic, nervous, and/or connective tissues or other cell populations with a low mitotic rate. These effects can occur anytime from 3 months to many years after radiation exposure. Most such problems occur between 9 and 24 months after completion of treatment; they rarely occur beyond 5 years. Most late effects caused by radiation do not rise to a level that meaningfully affects the patient’s quality of life. Typically, signs and symptoms such as chronic skin changes of epidermal telangiectasia and tanning, subdermal fibrosis, and mild-to-moderate soft-tissue fibrosis comprise the majority of radiation-induced late side effects. These side effects tend not to cause significant morbidity for the patient. As with acute reactions, late toxicity must be localized to the treatment field and is dependent on total dose, fractionation, and volume of the critical organs irradiated, and rare idiosyncratic patient response to radiation. In contrast to acute effects, most late-effect damage is irreversible. However, the use of tocopherol (vitamin E) and pentoxifylline has been reported to improve late-effect changes of soft tissue fibrosis in symptomatic patients. Hyperbaric oxygen therapy has also been shown to relieve several radiation-induced late side effects. Infrequently, significant permanent decrement in the patient’s quality of life can result. For example myelopathy, cataracts, xerostomia, gastrointestinal stricture, pulmonary fibrosis, lymphedema, nephropathy, osteoradionecrosis. and soft tissue scarring and/or necrosis are possible rare late outcomes, even in properly administered radiation therapy.
Laboratory data do not suggest that worse late toxicities of RT are related to host age. For example, several in vitro studies on fibroblasts, which are thought to be the principle cells involved in late radiation response, did not demonstrate a relationship between radiosensitivity and age. Animal studies of individual organ systems do not correlate aging with more severe late reactions, and several studies even suggest older animals show a greater resistance to the late effects of radiation. In Ruifrok et al., the latency period between spinal cord irradiation and the development of myelopathy was significantly longer in older versus younger rats. Another pair of experiments from separate laboratories, examining radiation-induced nephropathy, both demonstrated decreased renal radiosensitivity in older pigs and rats. In one clinical circumstance, the findings are less clear-cut. For CNS malignancies, some reports suggest elderly patients receiving brain irradiation to large treatment volumes are at greater risk of cognitive decline as a result of therapy. However, in this case, vascular comorbidities such as hypertension, diabetes, and atherosclerosis, with a higher incidence in the elderly, confound the causal analysis between age and toxicity. In addition, the conventional wisdom regarding cognitive changes associated with cranial irradiation has recently been augmented by the understanding that patients with CNS primary and metastatic disease often suffer preradiation neurocognitive problems. When baseline neurocognitive measures are made before RT, the imputed effects of RT fall away. Nevertheless, due care to limit the amount of brain irradiation in young and older patients remains a current tenet of good radiation oncology practice.
The concern over late toxicity may also be less relevant for some elderly patients with shorter life expectancy. In general, the risk of late complications can be reduced by decreasing the per-fraction dose. However, in such a case, the total course of radiation must be extended in order to achieve a high enough dose to control the tumor. For some patients, improving present quality of life is the higher priority over minimizing the possibility of late effects. In such cases, which are usually palliative, a shortened or hypofractionated course of RT is often effective and may provide the benefit of both symptom relief and abridged treatment days.
Comorbidities and Radiation Therapy in the Elderly
As previously described, clinical and laboratory studies do not suggest that aging alone affects the mechanisms of acute or late radiation response. However, aging is associated with comorbid illnesses, as well as with a decline in physiologic reserve. It is likely that these factors play the most relevant role in the selection of elderly patients for RT as well as their tolerance of it. Common medical conditions faced by the elderly such as hypertension, atherosclerosis, heart disease, and COPD are rarely, on their own, contraindications to RT. Rather, treatment and management decisions are influenced by a combination of factors including the anatomical region being irradiated, the volume of critical organs or structures in the treatment field, and the specific comorbidities and associated functional status of the patient. For example, because the older patient can be at increased risk for upper respiratory tract or urinary tract infections, special attention for the development of acute side effects in these organ systems may be warranted during their RT course. In another case, a patient with Parkinsonian tremor may pose a challenge because of his or her difficulty remaining still; however, appropriate immobilization and treatment field design usually obviates significant difficulties with delivery of RT in such patients. Finally, as elderly cancer patients already demonstrate high rates of fatigue and depression, minimizing treatment-related fatigue is particularly important for such patients. Studies suggest the RT-induced fatigue is less severe and lasting than its chemotherapy or combined modality counterparts, and with modern RT techniques further shrinking the irradiated volume and course of therapy, even greater gains have been observed. These examples highlight the heterogeneous composition of the elderly population, who despite comorbidities, with proper individualized assessment and treatment design, are still good candidates for RT. Considering the three major modalities of oncologic care, RT is often a reasonable option for the geriatric patient who may be unable to tolerate the physiologic stresses of surgery or chemotherapy.
Cutting-Edge Techniques in Radiation Therapy
The two major factors moderating the effectiveness and toxicity of RT are dose and the volume of tissue being irradiated. The biological effect of a particular total dose of radiation is a function of the dose per fraction, the fractionation scheme, and the total time over which the dose is delivered. Refinement in dose fractionation has been studied since radiation was first applied to the treatment of cancer. In the past decade, the use of advanced imaging technologies for both tumor target delineation and intratreatment target localization, introduction of sophisticated treatment planning software, and enhanced treatment delivery instruments have vastly improved the ability to precisely irradiate tumors while sparing normal tissues. Several of these techniques are valuable for the treatment of the elderly cancer patient.
Improved Targeting
Radiation fields were once as basic as a single treatment field (port) or uncomplicated anterior/posterior opposed (AP/PA) treatment fields with or without simple blocking utilized to shape the treatment beams. These approaches may still be appropriate field designs for specific cases; however, with the aid of improved imaging technology, especially CT, methods to deliver the dose to the target volume have dramatically improved the precision of radiation treatment. Intensity-modulated radiation therapy (IMRT), stereotactic radiosurgery (SRS), stereotactic radiotherapy (SRT), and stereotactic body radiation therapy (SBRT) are technologies used to treat tumors with the prescribed dose while at the same time dramatically minimizing irradiation of adjacent normal tissues to limit the short- and long-term side effects of treatment and maximize its therapeutic benefits.
A specific set of technologies called image-guided radiation therapy (IGRT) represents the latest advance in RT targeting. Utilizing imaging technologies of ultrasound, fluoroscopy, or CT combined with sophisticated localization techniques including stereoscopic shift technique, IGRT allows for daily localization of the treatment target, yielding increased precision of treatment and decreased normal tissue irradiation. IGRT is a critical aspect of improved targeting in RT.
IMRT
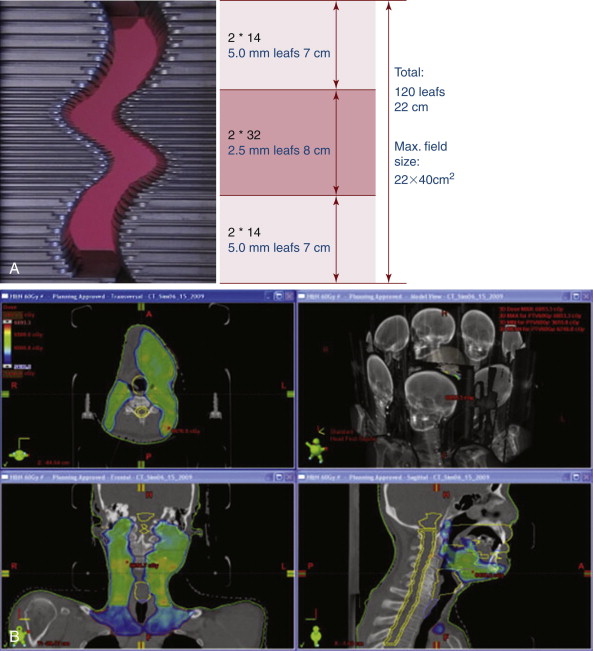
Traditionally, treatment planning decisions regarding beam angles and field shapes were made first during the isodose treatment planning process, followed by dosimetry calculations and modifications to achieve the prescribed dose to the intended target. This was called “forward” planning. Conversely, the initial step of IMRT defines the doses to the target volume and critical structures (also called organs at risk, OAR), followed by “inverse” treatment planning, which utilizes software that optimizes beam angles and shapes in order to produce the desired dose distribution. During the IMRT treatment, the patient, the treatment couch, and the beam all move, while at the same time the beam is mechanically spoiled or modulated. The result of this process is the mathematical equivalent of creating literally thousands of tiny microbeams aimed at the treatment target, producing a highly defined dose distribution irradiating the tumor while avoiding designated critical structures. ( Figure 7-2 )
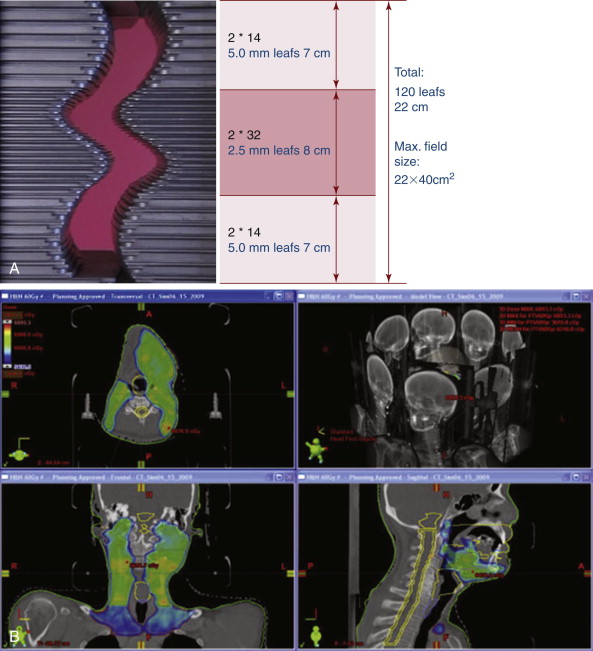
In the mid-1990s, use of IMRT began experimentally at a few institutions worldwide, but the past decade has seen a rapid increase in its application. IMRT has been shown to reduce rates of xerostomia in head and neck irradiation, permitted curative dose escalation in prostate cancer treatment while dramatically decreasing rectal and genitourinary treatment related morbidity, and provided a retreatment option for recurrent disease in previously irradiated areas, to name just a few examples of this technology’s significant benefits to patients. IMRT has emerged as the standard of care for a number of disease sites including prostate cancer, head and neck cancer, many CNS tumors, breast cancer, anal cancer, and esophageal cancer. Many other disease sites are actively under investigation to define the potential benefits of the precision of IMRT. IMRT’s potential to decrease treatment-related morbidity should not be underestimated. While to our knowledge no studies have examined the application of IMRT specifically for the geriatric population, the benefit of increased normal tissue sparing in elderly patients with multiple comorbidities is intuitively apparent.
SRS
Stereotactic radiosurgery was first described by neurosurgeon Lars Leksell and radiobiologist Bjorn Larsson in 1951 as a method to treat intracranial lesions, avoiding open surgery by utilizing a machine called the Gamma Knife. This technology, comprised of 201 Cobalt-60 sources focused on a single point, was developed to effectively treat a multitude of benign and malignant cranial lesions including arteriovenous malformations (AVM), acoustic neuromas, meningiomas, pituitary tumors, and primary and metastatic brain tumors. Today, a number of machines, including the Gamma Knife and modified or dedicated linear accelerators with SRS capability (e.g., Novalis TX, CyberKnife, XKnife, Trilogy, Synergy S), are able to treat cranial lesions.
The basic premises of SRS include: (1) a stereotactic frame of reference functioning to provide precise localization of an intracranial target; and (2) machinery capable of delivering one to five fractions of high dose radiation with very sharp dose fall-off gradients to minimize irradiation of surrounding tissues ( Figure 7-3 ). A treatment course of more than five fractions to an intracranial (or extracranial) tumor in which stereotactic localization is utilized in the delivery methodology is commonly referred to as stereotactic radiotherapy (SRT).
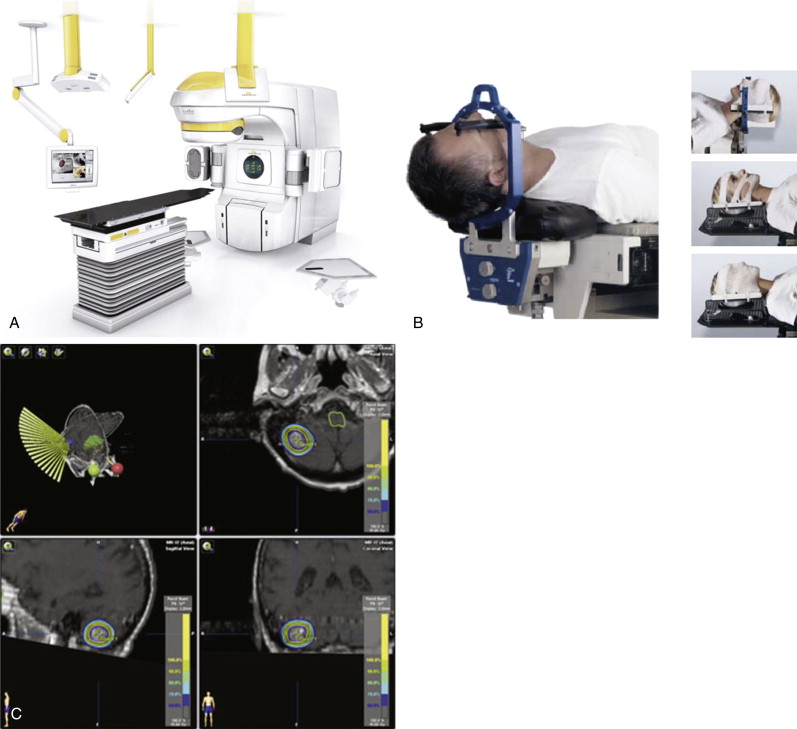
The use of SRS has become common in the elderly patient for the treatment of primary and metastatic brain tumors, meningiomas, AVMs, trigeminal neuralgia, and primary and recurrent pituitary tumors. The procedure is minimally invasive in nature and of short duration—usually one day. Customarily, a head frame was attached to the patient’s skull to ensure precision of treatment delivery, but recently technologies and techniques have been developed that allow completely noninvasive frameless treatments, which enhances patient acceptance of the procedure (see Figure 7-3 ). SRS is widely used for the treatment of brain metastases due to excellent local control rates and the possibility of avoiding the need for whole-brain radiation therapy (WBRT). The potential of avoiding WBRT, typically a 2 to 3 week course of treatment, may be important for the older patient facing difficulties with daily transportation or concerns of cognitive decline from RT.
SBRT
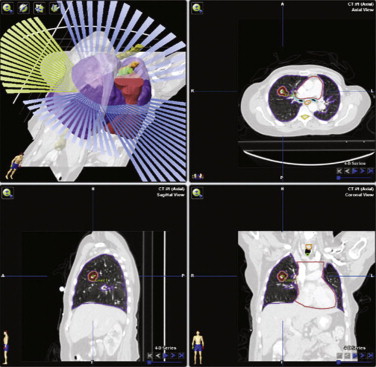
Treatment of extracranial tumors utilizing the precision of stereotaxis is known as stereotactic body radiotherapy (SBRT). Using only one to five fractions for the entire treatment course, it can be used to treat a number of anatomical sites effectively. Radiobiologically, the high dose and hypofractionated nature of SBRT is thought to exploit different mechanisms of cell damage than conventional RT by causing endothelial apoptosis and the upregulation of unique inflammatory cascades. SBRT’s truncated treatment course coupled with its comparable or superior tumor control as compared to standard fractionation makes it logistically beneficial for the elderly. SBRT is also a noninvasive alternative to surgery, which is of particular utility in the treatment of the older patient with significant comorbid diseases. For example, medically inoperable patients with early stage non-small cell lung cancer (with comorbidities of COPD, coronary artery disease, or cerebrovascular disease) who have historically been treated with conventional RT (with local control rates of only 30% to 50%) are now successfully managed with three to five SBRT treatments ( Figure 7-4 ). SBRT for early stage lung cancer has demonstrated 3-year local control and overall survival rates of 88% to 92% and 42% to 60%, respectively, establishing a superior alternative to conventional RT and a medically equivalent option to the surgical standard-of-care, lobectomy. The use of SBRT as the primary treatment for a number of tumors including prostate cancer, liver metastases, renal primary and metastatic disease, and pancreatic cancer is currently under investigation. For example, SBRT for early-stage prostate cancer was first examined prospectively by King et al. where five fractions of SBRT delivered every other day resulted in favorable PSA response without severe late rectal toxicities. While longer-term evaluation is necessary, this technique is a prime example of how dose and targeting modifications stemming from advancements in medical physics and discoveries in radiobiology provide a safe, effective treatment option for younger and older patients alike.
Brachytherapy
The surgical application of a radiation source placed within a body cavity (intracavitary) or implanted directly in the tissue or tumor itself (interstitial) is called brachytherapy. Many of the technical advantages of SRS and SBRT including dose escalation, conformality, and short duration of treatment were first achieved by brachytherapy. Interstitial treatment is invasive and often requires local or general anesthesia or conscious sedation. It may have the concomitant risk of bleeding and infection associated with a surgical procedure. Nevertheless, in certain situations, brachytherapy allows for convenient and low morbidity treatment of tumors in the elderly.
Brachytherapy is used to treat malignant diseases throughout the body including the brain, eye, head and neck, breast, lung, esophagus, biliary tract, endometrium, cervix, prostate, and soft tissues. Two examples highlighting the usefulness of brachytherapy in the elderly are its application in the two most common malignancies in the geriatric population, breast and prostate cancer.
Postlumpectomy management of breast cancer customarily requires whole breast external beam RT over 5 to 7 weeks. Alternatively, accelerated partial breast irradiation (APBI) using brachytherapy delivers treatment in 10 fractions over the course of five days, with early clinical experience demonstrating favorable results. ABPI is particularly applicable to the older cancer patient because of its short treatment duration. APBI compared to whole breast RT is being investigated prospectively by the large NSABP B-39 randomized trial, which is still in accrual.
Prostate “seed” interstitial brachytherapy involves the placement of radioactive isotopes directly into the prostate gland under ultrasound guidance. Due to the short half-life and lack of external penetration of the radioactive isotope, the radiation safety risks to medical personnel and the patient’s family members are de minimis . It is a 1-day procedure done on an outpatient basis. It has equivalent tumor control in low-risk prostate cancer patients and low rates of toxicity similar to those associated with other RT procedures or radical prostatectomy, which is usually not offered to patients older than 70 years.
Radiopharmaceuticals
Radiopharmaceuticals are used to treat systemic malignant disease. Given by oral, intravenous, or intraarterial routes, radioactive isotopes can be administered attached to pharmaceutical vehicles, as in radioimmunotherapy (RIT), or in an unattached soluble form, known as “unsealed source” RT.
Unsealed sources rely on the natural properties of the element to aggregate at the site of interest. For example, I-131 is absorbed and concentrated in follicular thyroid cells. As it decays, I-131 releases β particles, with a path length of 1 to 2 mm, which destroy normal thyroid and thyroid cancer cells. Other radionuclides including Sm-153 and Sr-89 are used to palliate widespread metastatic bone pain associated with metastatic breast, prostate, and lung cancer. These radioisotopes bind to hydroxyapatite, most actively at the tumor-bone interface of osteoblastic lesions, where they deliver therapeutic doses of radiation via beta decay. Myelosuppression is a possible side effect for all patients, which may be especially concerning in the aged, or when this treatment modality is used concurrently with other chemotherapeutics.
Radioimmunoglobulins are monoclonal antibodies linked to a radioisotope. Y-90 and I-131 are favored because of their short half-lives, beta decay, and stability in complex with the antibody. Radioimmunotherapy is most successful in the treatment of certain lymphomas. An international phase III randomized clinical trial reported that consolidation with Y-90 ibritumomab tiuxetan compared to no additional therapy after first-line induction for follicular lymphoma improved progression-free survival from 13.3 months to 36.5 months. Data from four clinical trials were pooled to examine the safety and efficacy of Y-90 ibritumomab tiuxetan in three age groups of non-Hodgkin lymphoma patients. Patients older than 70 years had similar rates of hematologic toxicity compared to the group of patients younger than 60, and rates and durations of response were similar in all age ranges.
Improved Targeting
Radiation fields were once as basic as a single treatment field (port) or uncomplicated anterior/posterior opposed (AP/PA) treatment fields with or without simple blocking utilized to shape the treatment beams. These approaches may still be appropriate field designs for specific cases; however, with the aid of improved imaging technology, especially CT, methods to deliver the dose to the target volume have dramatically improved the precision of radiation treatment. Intensity-modulated radiation therapy (IMRT), stereotactic radiosurgery (SRS), stereotactic radiotherapy (SRT), and stereotactic body radiation therapy (SBRT) are technologies used to treat tumors with the prescribed dose while at the same time dramatically minimizing irradiation of adjacent normal tissues to limit the short- and long-term side effects of treatment and maximize its therapeutic benefits.
A specific set of technologies called image-guided radiation therapy (IGRT) represents the latest advance in RT targeting. Utilizing imaging technologies of ultrasound, fluoroscopy, or CT combined with sophisticated localization techniques including stereoscopic shift technique, IGRT allows for daily localization of the treatment target, yielding increased precision of treatment and decreased normal tissue irradiation. IGRT is a critical aspect of improved targeting in RT.
IMRT
Traditionally, treatment planning decisions regarding beam angles and field shapes were made first during the isodose treatment planning process, followed by dosimetry calculations and modifications to achieve the prescribed dose to the intended target. This was called “forward” planning. Conversely, the initial step of IMRT defines the doses to the target volume and critical structures (also called organs at risk, OAR), followed by “inverse” treatment planning, which utilizes software that optimizes beam angles and shapes in order to produce the desired dose distribution. During the IMRT treatment, the patient, the treatment couch, and the beam all move, while at the same time the beam is mechanically spoiled or modulated. The result of this process is the mathematical equivalent of creating literally thousands of tiny microbeams aimed at the treatment target, producing a highly defined dose distribution irradiating the tumor while avoiding designated critical structures. ( Figure 7-2 )