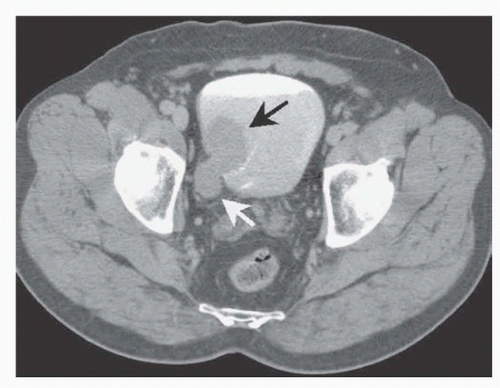
Types of Urinary Diversion
Urinary diversions may be divided into continent and incontinent. Incontinent urinary diversions or conduits involve the use of a segment of ileum or colon and, less commonly, a segment of jejunum. The distal end is brought to the skin, and the ureters are implanted into the proximal end. The patient wears a urinary collection appliance. The advantages of a conduit (ileal or colonic) are its simplicity and the reduced number of immediate and long-term postoperative complications. In most series, 13% of patients who undergo a cystectomy and urinary diversion of this type will have a significant complication that impacts on hospital stay or recovery. Generally, the distal ileum is used for the urinary conduit or reservoir; however, if it has been irradiated or is otherwise involved, one may select the right colon or a short segment of jejunum. The latter is the least desirable choice because electrolyte problems may be significant. On occasion, during exenterative surgery when an end colostomy is created, a segment of distal bowel is used, thus obviating the need for an intestinal anastomosis.
Continent diversions may be divided into two types: abdominal and orthotopic. Abdominal diversions require a continence valve, whereas an orthotopic neobladder depends on the urethral sphincter for continence. The reservoir is made of bowel that is fashioned into a globular configuration. In the abdominal type of continent diversion, the stoma is brought through the abdominal wall to the skin. The patient catheterizes the pouch every 4 hours. Orthotopic urinary diversions entail the use of bowel brought to the urethra, thus allowing the patient to void by Valsalva (
Fig. 39.2). Patients must have the facility to catheterize themselves, because it is mandatory in the abdominal continent diversion and occasionally necessary in the orthotopic reconstruction. The advantage of continent diversions is the avoidance of a collection device. The advantage of an orthotopic bladder over all other types of continent diversions is that it rehabilitates the patient to normal voiding through the urethra, often without the need for intermittent catheterization or the need to wear a collection device. Postoperative and long-term complications of continent diversion are increased over the conduit types of diversions. Indeed, in some series, postoperative complications range from 13% to 30%. Long-term metabolic complications are also increased.
Complications of Cystectomy and Urinary Diversion
The complications of all types of urinary diversion may be divided into three groups: metabolic, neuromechanical, and surgical.
Metabolic Complications of Urinary Intestinal Diversion. When the intestine is interposed in the urinary tract, there is the potential for a number of metabolic complications.
104 These may involve electrolyte abnormalities and altered drug metabolism, which may result in altered sensorium, infection, osteomalacia, growth retardation, calculi both within the reservoir as well as in the kidney, short bowel syndrome, cancer, and altered bile metabolism.
Depending on the segment used, different specific electrolyte abnormalities may occur. When the ileum and colon are used, hyperchloremic metabolic acidosis may result; when jejunum is used, hypochloremic or hyperkalemic metabolic acidosis may follow.
Hypokalemia is more common when the colon is used, whereas hypocalcemia is more common when the ileum and colon are used, and hypomagnesemia is more common when the ileum and the colon are used.
The most pervasive detrimental effect created by all urinary intestinal diversions is due to acidosis. Acidosis may result in electrolyte abnormalities, osteomalacia, growth retardation, altered sensorium, altered hepatic metabolism, renal calculi, and abnormal drug metabolism. In general, patients with normal renal function as well as normal hepatic function are less prone to acidosis and its complications.
Treatment for the metabolic acidosis is straightforward and can be accomplished with bicarbonate or with Bicitra solution, which is sodium citrate and citric acid. Polycitra, which is a combination of potassium citrate, sodium citrate, and citric acid, may also be employed. It has the advantage of supplying potassium, which, on occasion, is deficient. Chlorpromazine and nicotinic acid have been used to block the chloride bicarbonate exchanger, and thus lessen the potential for the acidosis.
Decreased renal function is seen in a majority of patients in the decade following a radical cystectomy, and choice of diversion does not predict the decline. Postoperative hydronephrosis, pyelonephritis, and uretero-enteric strictures represent factors that, if addressed, may mitigate the loss of function.
105Patients with conduits may have a 3% to 4% incidence of renal calculi over the long term. Those with reservoirs have up to a 20% incidence of calculi within the reservoir. The pathogenesis may be a metabolic alteration or infection, whereas reservoir stones are most commonly due to a surgical foreign body or mucus serving as a nidus.
There is a high incidence of bacteriuria in patients with either conduits or pouches, and the incidence of sepsis is 13%. There appears to be diminished antibacterial activity of the intestinal mucosa, with the immunoglobulins, which are normally secreted by the mucosa, being altered. In addition to this, when the bowel is distended, there can be a translocation of bacteria from the lumen into the bloodstream.
Because the intestine is interposed in the urinary tract, drugs that are eliminated unchanged from the body through the kidney and have the potential to be reabsorbed by the gut can in fact result in significant alterations in metabolism of that drug. Patients with a urinary diversion, when given systemic chemotherapy, have a higher incidence of complications and are more likely to have their chemotherapy limited when compared with patients without diversion who receive the same drugs and dose.
106The loss of the distal ileum may result in vitamin B12 malabsorption, which then manifests itself as anemia and neurologic abnormalities. Bile salt malabsorption may occur and result in diarrhea. Loss of the ileocecal valve may result in diarrhea with bacterial overgrowth of the ileum and malabsorption of vitamin B12 and fat-soluble vitamins A, D, E, and K. Loss of the colon may result in diarrhea and bicarbonate loss.
Neuromechanical Complications. Neuromechanical complications may be of two types: atonic, resulting in an atonic segment with urinary retention, and hyperperistaltic contractions. The latter is relevant in continent diversions, as this may result in incontinence and a low-capacity reservoir.
Surgical Complications. There are a number of complications that occur following any major surgical procedure, which include thrombophlebitis, pulmonary embolus, wound dehiscence, pneumonia, atelectasis, myocardial infarction, and death. Complications specific to cystectomy and urinary diversion are divided into short term and late. The short-term complications include acute acidosis (16%), urine leak (3% to 16%), bowel obstruction or fecal leak (10%), and pyelonephritis (5% to 15%). The longer term complications include ureteral or intestinal obstruction (15%), renal deterioration (15%), renal failure (5%), stoma problems (15%), and intestinal stricture (10% to 15%).
107,108The morbidity of salvage cystectomy for a recurrence following bladder sparing chemoradiation has also been described and appears acceptable when compared to primary cystectomy series.
109
Selective Bladder-Preserving Approaches
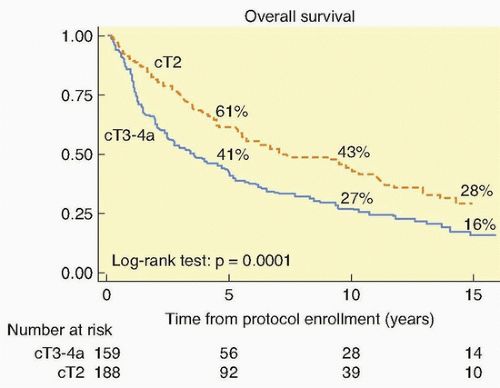
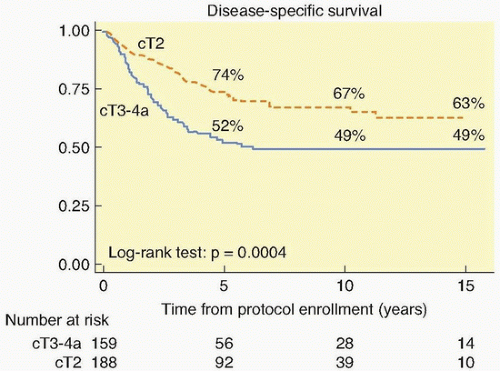
The treatment options for muscularis propria-invasive bladder tumors can broadly be divided into those that remove the bladder and those that spare it. In the United States, a radical cystectomy with pelvic lymph node dissection remains the standard method used to treat patients with this tumor. Several reports from North America and Europe have described long-term results using multimodality treatment of muscularis propria-invading bladder cancer, with appropriate safeguards for early cystectomy should this treatment fail. For bladder-conserving therapy to be more widely accepted, this treatment approach must have a high likelihood of eradicating the primary tumor, must preserve good organ function, and must not result in compromised patient survival. It does appear that, for selected patients, bladder sparing therapy with salvage cystectomy reserved for tumor recurrence represents a safe and effective alternative to immediate radical cystectomy.
110Successful bladder-preserving approaches have evolved during the past 3 decades. They began with the use of radiation therapy but expanded when the National Bladder Cancer Group first demonstrated the safety and efficacy of cisplatin as a radiation sensitizer in patients with muscle-invasive bladder cancer that was unsuitable for cystectomy.
111 The long-term survival with stage T2 tumors (64%) and stage T3 to T4 tumors (22%) was encouraging. This was validated by the National Cancer Institute-Canada randomized trial of radiation (either definitive or precystectomy) with or without concurrent cisplatin for patients with T3 bladder cancer, which showed a significant improvement in long-term survival with pelvic tumor control (67% versus 47%) in the patients who were assigned cisplatin.
112 Additional single-institution studies showed that the combination of a visibly complete TURBT followed by radiation therapy or radiation therapy concurrent with chemotherapy safely improved local control.
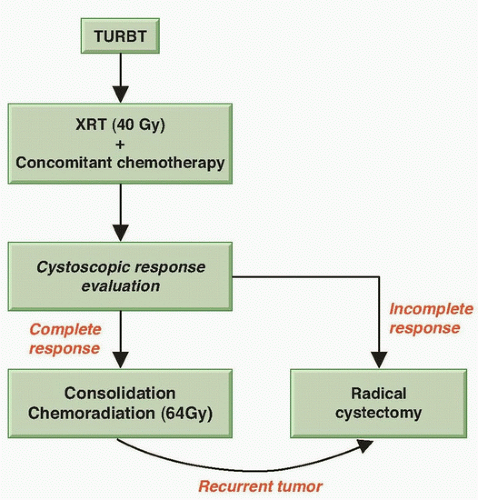
113,114 These findings led the RTOG to develop protocols for bladder preservation beginning with a TURBT of as much of the tumor as is safely possible, followed by the combination of radiation with concurrent radiosensitizing chemotherapy. One key to the success of such a program is the selection of patients for bladder preservation on the basis of the initial response of each individual patient’s tumor to therapy. Thus, bladder conservation is reserved for those patients who have a clinical CR to concurrent chemotherapy and radiation. A prompt cystectomy is recommended for those patients whose tumors respond only incompletely or who subsequently develop an invasive tumor (
Fig. 39.3). Up to 30% of the patients entering a potential bladder-preserving protocol with trimodality therapy (initial TURBT followed by concurrent chemoradiation) will ultimately require a salvage radical cystectomy.
For over 2 decades, the Massachusetts General Hospital (MGH), the RTOG, and several centers in Europe have evaluated in phase II and III protocols concurrent chemoradiation plus neoadjuvant or adjuvant chemotherapy (
Table 39.3). Radiosensitizing drugs studied in these series, either singly or in various combinations, include cisplatin, carboplatin, paclitaxel, 5-fluorouracil (5-FU), mitomycin C,
and gemcitabine.
113 The first RTOG study of patients treated with once-daily radiation treatment and concurrent cisplatin yielded a 5-year survival of 52% (42% with intact bladder).
115 RTOG studies 8802 and 8903 used methotrexate, cisplatin, and vinblastine (MCV) chemotherapy as neoadjuvant treatment.
116 In the latter study, the neoadjuvant therapy was tested in a randomized fashion.
117 No improvement was seen in survival or in local tumor eradication as a result of neoadjuvant therapy, although the trial was closed early and underpowered to give a definitive answer. The toxicity of the MCV arm was considerable, with only 67% of patients able to complete the planned treatment. The use of contemporary neoadjuvant chemotherapy (dose-dense methotrexate, vinblastine, adriamycin, cisplatin [ddMVAC] or gemcitabine and cisplatin [GC]) regimens with appropriate supportive therapy in well-selected bladder-sparing patients may warrant further investigation.
Other studies from Paris and Germany have reported their large experience with bladder sparing.
118,119 The CR rate in the German study was 72%, and local control of the bladder tumor after the CR without a muscle-invasive relapse was maintained in 64% of the patients at 10 years. The 10-year disease-specific survival was 42%, and more than 80% of these survivors preserved their bladder. This series reported the sequential use of radiation with no chemotherapy (126 patients), followed by concurrent cisplatin (145 patients), then concurrent carboplatin (95 patients), and finally concurrent cisplatin with 5-FU (49 patients). The CR rates in these four protocols were 51%, 81%, 64%, and 87%, respectively.
120,121 The 5-year actuarial survival with an intact bladder in these studies was 38%, 47%, 41%, and 54%, respectively. These results strongly suggest that radiochemotherapy, when given concurrently, is superior to radiation therapy alone; that carboplatin is less radiosensitizing than cisplatin; and that cisplatin plus 5-FU may be superior to cisplatin alone.
The RTOG protocols have subsequently explored both twicedaily radiation therapy and novel radiosensitization using cisplatin with or without 5-FU or paclitaxel.
62,122,123,124 Complete response and bladder preservation rates are consistently high, with no one regimen clearly superior.
62Gemcitabine has been also tested in bladder-treatment protocols. In a phase I trial from the University of Michigan, 23 patients, mostly T2, were treated with gemcitabine and concurrent daily radiation. At a median follow-up of 5.6 years, an impressive 91% CR rate was observed, and the 5-year actuarial estimates of survival include a bladder-intact survival of 62%, an overall survival of 76%, and a disease-specific survival of 82%.
125 A phase II study from the United Kingdom of 50 patients treated with concurrent weekly gemcitabine and hypofractionated radiation reported an 88% complete endoscopic response rate, a 3-year overall survival of 75%, and cancer-specific survival of 82%.
126 Twice weekly low-dose gemcitabine was recently evaluated as a radiosensitizer with daily radiation in protocol RTOG 0712.
Cisplatin is not always an ideal drug for bladder cancer patients, because it may cause impaired renal function in many. A British group observed high response rates using the combination of 5-FU
and mitomycin C with pelvic radiotherapy.
127 These results led to the phase III Bladder Cancer 2001 (BC2001) trial, in which 360 patients with muscle-invasive bladder cancer were randomized to either radiotherapy alone or to radiotherapy with concomitant 5-FU and mitomycin C chemotherapy. Local-regional disease-free survival was superior for those patients receiving chemotherapy (67% versus 54% at 2 years; hazard ratio [HR] 0.68,
p = 0.03 with median follow-up of 70 months). Survival at 5 years was higher with chemoradiotherapy (48% versus 35%), but did not reach statistical significance (HR 0.82;
p = 0.16).
128