Specimen type
Patient group
–
Ig CTF ng/mL
n
Aver
sd
min
max
Serum
Normal
25
322.5
173.4
55.5
778.8
Serum
Liver disease
23
1141.2
1514.3
293.8
6854.7
Serum
Breast cancer
5
794.6
260.7
340.7
1140.0
Serum
Pancreatic cancer
10
581.2
430.5
293.2
1330.7
–
–
–
–
–
–
–
Plasma
Normal
25
727.5
343.0
290.2
1723.4
Plasma
Anti TNF α therapy
8
404.1
130.1
194.1
601.0
Plasma
Pre-diabetic (IGT)
119
349.5
268.1
95.1
2346.3
Plasma
Pre-diabetic (IFG)
100
335.3
256.4
99.0
2346.3
Plasma
Diabetic
24
268.0
141.1
91.3
645.7
–
–
–
–
–
–
–
Whole blood
Normal
101
1234.1
651.9
178.6
4758.9
Whole blood
Inflammation
13
402.1
173.6
145.2
713.2
Whole blood
Immunosuppressant
20
566.0
361.1
160.7
1427.1
Whole blood
Pre-diabetic
48
527.0
195.6
250.9
1055.2
Whole blood
Diabetic
63
222.3
97.3
51.6
444.1
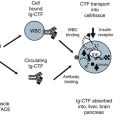
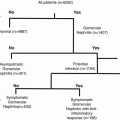
The values for Ig-CTF were next compared with patients undergoing glucose and HbA1c testing. The Ig-CTF plasma values for patient with pre diabetes and diabetes were significantly lower than patients with normal glucose tolerance (P > 0.01). Overnight fasting did not impact Ig-CTF values in health donors or diabetics with all values within 9–14 % of non-fasting values. The Ig-CTF values did not change during the 120 min following glucose administration. Values did not change over 45 days. This is consistent with the 14 day plasma half-life of immunoglobulin (Hinton et al. 2006). Values did changed in a patients who underwent significant a weight change (>20 % drop) after 45 days. This contrasts the rapid changes of free CTF measured in animals during the 120 min following glucose administration (See Chap. 5). Ig-CTF appears to be more a chronic phase marker.
6.3.2 Correlation to Inflammation
The values for Ig-CTF measured shown for normal and diabetic donors were in cases lacking inflammation. Inflammatory conditions were ruled out by a CPR <5 mg/dl or CBC of <10 white blood cells/mL. These cut-off do not rule out mild elevation of inflammation. The patients with significant inflammation, as indicated a CRP and CBC positive, did exhibited a significantly lower Ig-CTF (P > 0.01) than normal patients (Table 6.1). This decrease was of a similar magnitude to a decrease in the Ig-CTF seen in diabetes.
Patients using anti-inflammatory therapies such as Remicade® (Infliximab) exhibited suppressed Ig-CTF values. Transplant patients on immunosuppressants also had suppressed Ig-CTF values. The impact of anti-TNF alpha inhibitor drugs, would be expected to prevent CTF formation by inhibition of TACE. Others drug expected to impact the assay would include adalimumab (Humira), certolizumab pegol (Cimzia), golimumab (Simponi), or etanercept (Enbrel). Immunosuppressants would also be expected to lower immunoglobulin levels and suppress Ig-CTF formation.
Patients who had diseases that would be expected to increase blood immunoglobulin levels, also had changes in the values for Ig-CTF. Liver diseases represent a significant inflammatory disease group. Liver diseases (Hepatitis & cirrhosis) elevate serum immunoglobulin. Values for Ig-CTF were significantly increased with auto-immuno hepatitis patients having the highest values (Table 6.2). Patients with leukemia, multiple myeloma, and lymphoma were excluded as these cancers would clearly elevate immunoglobulin levels. However, cancer is generally associated with greater blood immunoglobulin levels and many patients with breast and pancreatic carcinomas did exhibited increased Ig-CTF values.
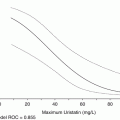
Table 6.2
The predictive values of CTF-Ig for in gestational study
Study 1 | Study 2 | |
|---|---|---|
TN | 95 | 110 |
FN | 0 | 10 |
FP | 7 | 10 |
TP | 64 | 84 |
Sens | 100.0 % | 89.4 % |
Spec | 93 % | 92 % |
PPV | 90 % | 89 % |
NPV | 100 % | 92 % |