Bronchioloalveolar Carcinoma/Adenocarcinoma-in-Situ
Philip T. Cagle
Keith M. Kerr
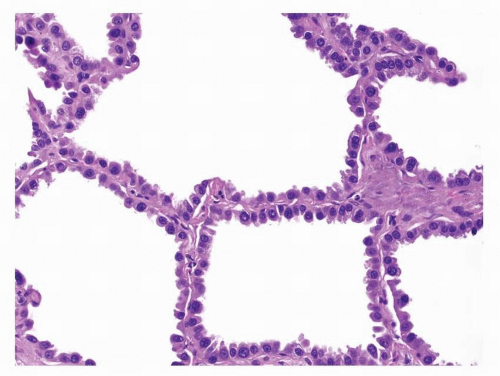
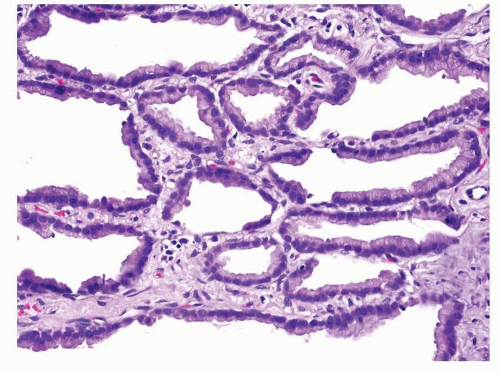
First described by Averill Liebow in 1960,1 bronchioloalveolar carcinomas (BACs) were thought to be derived from the peripheral pulmonary epithelium with tumor cells resembling Clara cells, goblet cells, or type II pneumocytes. The characteristic histologic feature was growth of the tumor cells along the surface of intact alveolar septa, referred to as lepidic growth. Prior to 1999, both adenocarcinomas with pure lepidic growth pattern and adenocarcinomas with a prominent BAC component mixed with invasive components were diagnosed as BACs and included in published studies as BACs. Currently, BAC is histologically defined as an adenocarcinoma-in-situ (AIS) that grows in a lepidic manner, replacing the original pulmonary epithelial lining, along intact alveolar septa without invasion into the underlying stroma, pleura, or lymphovascular spaces ( Figs. 4-1,4-2 and 4-3).2,3,4,5,6,7,8,9,10,11,12,13,14 and 15
BACs are most frequently nonmucinous, but mucinous and mixed nonmucinous and mucinous types are described. Most of the preceding remarks apply to nonmucinous BAC rather than mucinous BAC. The nonmucinous and mucinous variants have critical differences in their histopathology, gross, radiology, molecular pathology, and clinical features and are, therefore, discussed separately.2,3,4,5,6,7,8,9,10,11,12,13,14 and 15 Traditionally, the nonmucinous tumor cells are thought to resemble Clara cells or type II pneumocytes and mucinous tumor cells are thought to resemble goblet cells. Most of this chapter focuses on the nonmucinous type, which is more frequent than the mucinous type (>2/3 of BAC) and is generally considered to be the form that fits into the progression scheme from atypical adenomatous hyperplasia (AAH) to BAC (adenocarcinoma-in-situ) to invasive peripheral adenocarcinoma (see Chapter 27).2,3,4,5,6,7,8,9,10,11,12,13,14 and 15 The frequency with which an individual pathologist may see one type rather than the other will, however, depend on the country in which he or she practices and the local protocols relating to lung cancer screening and thoracic surgical practice.
This current definition of BAC was first adopted in the 1999 World Health Organization classification,2 was continued in the 2004 World Health Organization classification,3 and is based largely on the study of small, peripheral adenocarcinomas by Noguchi et al.16 Noguchi et al.’s original study16 and subsequent studies17,18,19,20,21,22,23 and 24 support the concept of progression from BAC or AIS to minimally invasive adenocarcinomas that have a prominent BAC component. Noguchi Type A tumors consist of pure BAC, Type B tumors consist of BAC with alveolar collapse and fibrosis, and Type C tumors consist of a predominantly BAC pattern with foci of invasion and associated fibroblastic proliferation. Noguchi Type A and B tumors have a 5-year survival rate of 100%, whereas Noguchi Type C tumors have a 5-year survival rate of 75%.16 Some experts are proposing that the term bronchioloalveolar carcinoma be discarded and that pure localized BAC tumors (Noguchi Type A and B) be designated AIS. In this proposed classification, adenocarcinomas with minimal invasion would also be recognized (see Chapter 5).14
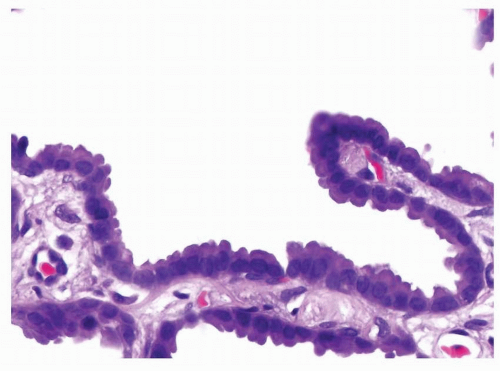 FIGURE 4-3 High power of Figure 4-2 demonstrates that the neoplastic columnar cells are cytologically bland and comparatively homogeneous. They are arranged adjacent to each other covering the surface of the septum with a row of single cells in a lepidic pattern without invasion of the septum or other structures. |
Using the current definition of BAC as strictly noninvasive or in-situ carcinoma, only 2% to 6% of non-small cell carcinomas are pure BACs. About 40% to 50% of mixed pattern non-small cell carcinomas have a BAC component, however, and in 7% to 16% this is the dominant pattern in the tumor.5,25,26 Surgical resection results in a 5-year survival rate of 100% for small, solitary
peripheral BACs, consistent with their in-situ status.6,16 Since invasion must not be present in order to meet the current diagnostic criteria for BAC, a final diagnosis of true BAC cannot be made on a small transbronchial biopsy, needle core biopsy, or cytology specimen. This is because the entire tumor is not sampled in these limited specimens and, therefore, invasion elsewhere in the tumor cannot be excluded.2,3,4,5,6,7,8,9,10,11,12,13,14 and 15
peripheral BACs, consistent with their in-situ status.6,16 Since invasion must not be present in order to meet the current diagnostic criteria for BAC, a final diagnosis of true BAC cannot be made on a small transbronchial biopsy, needle core biopsy, or cytology specimen. This is because the entire tumor is not sampled in these limited specimens and, therefore, invasion elsewhere in the tumor cannot be excluded.2,3,4,5,6,7,8,9,10,11,12,13,14 and 15
BAC has some unique demographic features compared to other lung cancers. Although approximately 75% of BACs are associated with tobacco smoking, about 25% of BACs occur in never smokers, a greater percentage than with any other lung cancer cell type. In addition, a majority (up to 63%) of patients with BAC are women.4,10,14,17 Caution is required, however, in interpreting the literature in this area, since published data on BAC, even post-2000, do not necessarily concern tumors fulfilling the current strict definition of BAC.
NONMUCINOUS BRONCHIOLOALVEOLAR CARCINOMA, GROSS FEATURES
Nonmucinous BACs make up at least 60% to 75%, possibly almost 100% of true BAC.2,3,4,5,6,7,8,9,10,11,12,13,14 and 15 Nonmucinous BACs are peripheral, ivory to tan lesions measuring a few millimeters to around 2 cm in size. It is extremely unusual to find a lesion over 2 cm in diameter that does not have invasion. Most are solitary, but they are occasionally multifocal. Nonmucinous BAC may have a spongy texture or be poorly defined from surrounding parenchyma due to the preservation of airspaces by tumor cells growing in a lepidic pattern along intact alveolar septa. In well-preserved specimens the alveolar spaces can be seen on the lesion cut surface with the naked eye. Collapse of fibrotic alveolar septa or a central scar may make the tumor firmer, but, by definition, invasion is not present in pure BAC. Therefore, there is no desmoplastic reaction, necrosis, or hemorrhage. Subpleural BAC may, however, have puckering of the overlying pleura.2,3,4,5,6,7,8,9,10,11,12,13,14 and 15
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree