Bronchial Squamous Dysplasia and Carcinoma In Situ
Keith M. Kerr
This chapter will review squamous dysplasia and carcinoma in situ (SD/CIS), recognized in the WHO classification of lung tumors as precursor lesions for the development of bronchogenic squamous cell carcinoma.1 The evolution, morphology, differential diagnosis, and molecular biology of these lesions will be considered, as well as some comment on the clinical relevance of the lesions, particularly with respect to lung cancer screening. SD/CIS are regarded as preinvasive lesions since they have some, but not all, of the morphological features of malignancy; by definition, they lack hallmark invasion yet have the potential for its development. Occasionally, these lesions are referred to as preneoplastic and their importance is that the relative risk of these lesions becoming invasive is high. Almost certainly, SD/CIS does not arise de novo from bronchial respiratory epithelium. It is hypothesized that SD transforms into CIS, but SD itself also has likely precursor lesions.
Animal models have been extensively used to study the process of malignant transformation in bronchial epithelium. While many of these models do not absolutely reflect the situation in the human bronchial tree, primarily since, setting aside the obvious species differences, experiments have tended to involve the use of much higher doses of irritants or carcinogens and concern models of disease with a time course orders of magnitude shorter that that seen in the human, they have, nonetheless, given useful insight into what probably happens in the human airway2 A consistent finding in many of these models is the initial induction of inflammation and respiratory cell injury, followed by a proliferative/reparative reaction. Metaplasia occurs soon after; usually, this is squamous metaplasia. Ultimately dysplasia supervenes.
BRONCHIAL CARCINOGENESIS
It is very likely that a similar sequence of events occurs in the human. As will be discussed later, the most important etiological factor in the development of SD/CIS is tobacco smoke. As well as containing 50 to 60 recognized carcinogens, there are around 4,000 other chemical substances in tobacco smoke, some of which are responsible for chronic irritation of the tracheobronchial epithelium.3,4 This irritation leads to a number of morphological changes, which are described below. These changes include mucous cell hyperplasia, basal cell hyperplasia, and squamous metaplasia; all may be regarded as reactive or adaptive changes in the respiratory mucosa, leading to an epithelium better able to cope with the prevailing “toxic” environment. While a role for mucous cell hyperplasia in the evolution of SD/CIS is not clear, basal cell hyperplasia and squamous metaplasia are both implicated as “precursors” for the development of SD. It is likely that these reactive lesions represent epithelia, which have in some ways “begun” the molecular and morphological evolution toward malignancy. The emergence of a phenotypically malignant cell population is the result of the accumulation, in that cell population, and most critically in the stem cells, of a number of key genetic changes or abnormalities. Lesions such as SD and CIS are a morphological representation of the accumulation of many of these genetic changes; it is presumed that a few more are required for the fully invasive malignant phenotype to evolve. It has been estimated that between 3 and
12 critical genetic changes must occur, in a specific order, for malignancy to develop.5 In fact, as discussed below, many more changes than this actually accumulate as dysplasia and CIS evolve and it is crucial that none of the accumulated changes are fatal to the cells affected. The chances of the required genetic changes occurring in the correct cells (cells with stem-like properties), in the correct sequence, are actually very small. Cancer is a “statistical” disease. Doll correctly described the evolution of cancer as “…largely a matter of luck: bad luck….”6
12 critical genetic changes must occur, in a specific order, for malignancy to develop.5 In fact, as discussed below, many more changes than this actually accumulate as dysplasia and CIS evolve and it is crucial that none of the accumulated changes are fatal to the cells affected. The chances of the required genetic changes occurring in the correct cells (cells with stem-like properties), in the correct sequence, are actually very small. Cancer is a “statistical” disease. Doll correctly described the evolution of cancer as “…largely a matter of luck: bad luck….”6
Following the concept of field carcinogenesis, which applies well in the case of the bronchus, and indeed the peripheral lung epithelium (see Chapter 27), multiple foci of preinvasive change are usually present in the affected tracheobronchial tree.7 Each lesion probably represents a patch of altered epithelium which has accumulated some but not all of the crucial genetic changes required for the full malignant phenotype. Each patch may well represent independent clonal expansion of an altered stem cell population; such cells are believed to reside in the bronchial gland ducts and at intracartilagenous boundaries in the large airways mucosa. More patches will exist with fewer genomic changes and therefore less morphological abnormality; fewer areas will have evolved genetically further and show more severe atypia or even CIS. In some patients, one of these lesions “makes it” to invasive carcinoma; occasionally, patients develop two or even more foci of independently evolving malignancy (synchronous primary tumors). Some of these genetic changes, which are consistently found in SD/CIS but less often in basal cell hyperplasia and squamous metaplasia, may also be found in morphologically normal respiratory epithelium of tobacco smokers, as discussed below. This is presumably evidence that the earliest genetic changes precede the evolution of morphological change.
Bronchial carcinogenesis has been associated with exposure to ionizing radiation or certain chemicals, and the role of air pollution is still debated, but more is known about tobacco-related carcinogenesis. Of the numerous carcinogens found in tobacco smoke, the two most important groups are the polycyclic aromatic hydrocarbons and the N-nitrosamines.3,4 While the former have been particularly associated with bronchial carcinogenesis and N-nitrosamines, especially a substance known as NNK, with peripheral lung adenocarcinogenesis, the effects of these compounds are not anatomically exclusive. Many of the compounds in tobacco smoke actually require chemical modification to become active carcinogens. It is something of a paradox that xenobiotic metabolizing enzymes (XMEs) that have a physiological role in detoxification of foreign chemical compounds may actually activate procarcinogens in tobacco smoke. Other enzymes are involved in further chemical modification of these activated carcinogenic compounds, inactivating them and facilitating their elimination. The ultimate “carcinogenic effect” of particular tobacco carcinogens will, therefore, depend on the relative efficiency of the so-called type 1 XMEs, such as many of the cytochrome P450s which activate procarcinogens and the type 2 XMEs, including glutathione-S-transferases which detoxify active carcinogens. If activation is efficient and detoxification is poor, carcinogenesis is more likely; vice versa would see a situation mitigating against tumor development. This is a simplification of a complex process involving many different carcinogens and numerous different enzymes. Many of these enzymes exist in different isoforms. Some isoforms are more or less efficient than others in their catalytic actions. It follows that an individual’s ability to detoxify or chance of suffering cytogenetic damage from tobacco carcinogens will depend on the particular enzyme gene polymorphisms he or she has inherited. There is growing evidence of particular combinations of polymorphisms (genotypes), which are associated with greater risk of developing lung cancer.8 Polymorphisms of the nicotinic acetylcholine receptor gene cluster are also associated with an apparent variable risk in developing lung cancer. These polymorphisms appear to determine variability in nicotine addiction and may stimulate greater tobacco consumption in some individuals.
PREINVASIVE LESIONS OF BRONCHIAL MUCOSA
Goblet Cell Hyperplasia
Some have suggested, largely on the basis of animal studies, that hyperplastic mucinous cells in the bronchial epithelium may transform into squamous metaplasia and dysplasia; at best, this is speculation. Certainly, this lesion is one of the adaptive changes seen in tobacco smokers and one
of the key changes leading to the mucous hypersecretory state which defines chronic bronchitis (Fig. 26-1). Goblet cell hyperplasia is manifest in the central cartilaginous airways as small foci where, instead of occasional goblet cells admixed with other differentiated columnar cells in normal epithelium, there are patches of numerous goblet cells crowded together, sometimes giving a slightly raised or tufted appearance to the affected epithelium. There is no cytological atypia.
of the key changes leading to the mucous hypersecretory state which defines chronic bronchitis (Fig. 26-1). Goblet cell hyperplasia is manifest in the central cartilaginous airways as small foci where, instead of occasional goblet cells admixed with other differentiated columnar cells in normal epithelium, there are patches of numerous goblet cells crowded together, sometimes giving a slightly raised or tufted appearance to the affected epithelium. There is no cytological atypia.
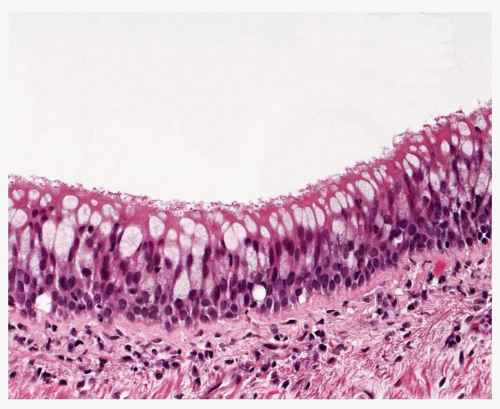 FIGURE 26-1 Goblet cell hyperplasia. The mucosa in this lobar bronchial mucosa has an excess of goblet cells (compare with Fig. 26-2). |
Basal Cell Hyperplasia
Basal cell hyperplasia is defined by the presence of three or more layers of basal cells in the respiratory epithelium (Fig. 26-2). Sometimes, there may be many more layers of cells almost completely replacing the epithelium but leaving a columnar cell layer on the surface. These cells are regular with small, round to oval nuclei and there is no atypia. Despite the hyperplastic terminology mitoses are very uncommon. Tangential cutting of the bronchial epithelium can give a false impression of basal cell hyperplasia, but in such a case, the basement membrane is also apparently thickened and the surface columnar cells do not appear vertically sectioned. It may be very difficult to discriminate between basal cell hyperplasia and some patterns of mild dysplasia. The basal cell layer may be demonstrated using immunohistochemistry and antibodies to p63 protein or cytokeratin 5/6.
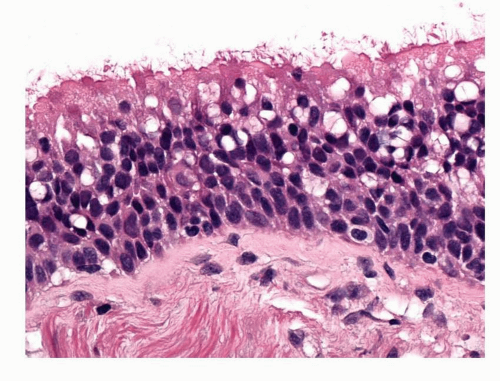 FIGURE 26-2 Basal cell hyperplasia. Increased layers of basal cells (compare with Fig. 26-1). Basal cell nuclei are slightly enlarged but there is no significant atypia. |
SQUAMOUS METAPLASIA
This is recognized when the bronchial epithelium is replaced by a differentiated squamous epithelium comprising basal cells, a zone of larger prickle cells and superficial flattening of cells with keratinization (Fig. 26-3). As with basal cell hyperplasia, there should be no cytological atypia. In the proposed evolution of SD and CIS, squamous metaplasia is seen as the stage immediately preceding SD. Squamous metaplasia is an adaptation by the airway epithelium to chronic irritation and as well as tobacco smoke; it has been associated with air pollution, irradiation, smoking marijuana, and many chronic inflammatory or infective situations involving airways or lung cavities.2,9 Long-standing airway intubation may lead to squamous metaplasia at points of trauma, it is associated with vitamin A deficiency, and it is quite common for a thin layer of squamous metaplastic epithelium to cover relatively long-standing endobronchial tumors such as carcinoid tumors, lipomas, or endobronchial hamartomas (Fig. 26-4). Squamous metaplasia may also be seen in alveolated lung in the context of organizing diffuse alveolar damage or usual interstitial pneumonia.
Squamous metaplasia as described above is not an obligate precursor for SD. Fully mature squamous metaplasia is not so common. More often, evidence of squamous differentiation
(prickle cells) is seen in expanded zones of basal cell hyperplasia, surmounted by differentiated columnar cells. This could be considered immature squamous metaplasia and dysplasia may occur in this setting without full-thickness squamous change.
(prickle cells) is seen in expanded zones of basal cell hyperplasia, surmounted by differentiated columnar cells. This could be considered immature squamous metaplasia and dysplasia may occur in this setting without full-thickness squamous change.
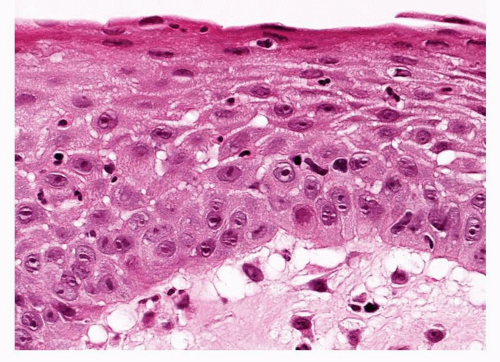 FIGURE 26-3 Complete, full-thickness squamous metaplasia of the bronchial mucosa. A relatively unusual lesion, it shows a marked prickle cell layer and superficial maturation with keratinization. |
SQUAMOUS DYSPLASIA AND CARCINOMA IN SITU
Much of the early work on these lesions and their association with bronchogenic carcinoma and tobacco smoking came from the autopsy studies of Auerbach et al.10 Interest in SD/CIS has increased recently as a result of the expanding use of autofluorescence bronchoscopy (AFB) to detect and localize bronchial mucosal abnormality, as discussed below.
The strongest known etiological factor for SD/CIS is tobacco smoking.11 Other studies have been carried out on patient cohorts exposed to airborne irradiation (radon—uranium miners) or chemicals such as chromates but much of these data were confounded by patients also smoking.12 There is some evidence of a dose-response relationship between amount smoked and the number and grade of dysplastic lesions that may be found. It is somewhat controversial but smoking cessation may be associated with lesion regression. Associations with air pollution are difficult to prove. SD/CIS is commoner in males than females; this may be related to gender differences in smoking habits. The macroscopic features of SD/CIS are rarely appreciated, either by pathologists or bronchoscopists. Most lesions are invisible in pathological material, partly due to the subtle changes they induce but also because of the quality of material and the intercurrent pathology that is often present. SD/CIS lesions are most often found on the spurs of airway carinae. Lesions may cause loss of the rugal folds of the mucosa, opacification and roughening of the surface and occasionally result in slightly elevated plaques. Extensive disease can, at least theoretically, lead to retention pneumonia if there is replacement of enough normal ciliated respiratory epithelium to interrupt the mucociliary flow. Lesions are generally quite small. CIS lesions have been described at between 2 and 17 mm in diameter with a mean diameter of around 8 to 9 mm. SD lesions tend to be smaller, mostly measuring around 1.5 to 5 mm in greatest dimension (Fig. 26-5).13,14
Histologically, there are four grades of disease defined within the WHO classification of SD/CIS; mild, moderate and severe dysplasia, and carcinoma in situ.1 This is, thus, a relatively complex classification, and though following those originally proposed for other organ sites such as the cervix, there have been suggestions that a two-tier system of low- and high-grade dysplasia would be better. The classification is based upon the identification of architectural and cytological changes distributed within a full-thickness squamous epithelium. All the possible features may not necessarily be present and may vary from microscopic field to field within a lesion.
The dysplastic epithelium is notionally divided into lower (basal), middle, and upper thirds for the purposes of assessing the distribution of changes.
The dysplastic epithelium is notionally divided into lower (basal), middle, and upper thirds for the purposes of assessing the distribution of changes.
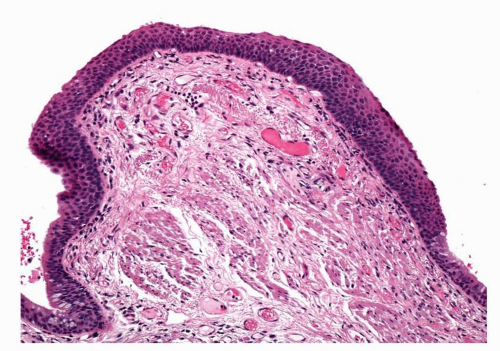 FIGURE 26-5 A small, localized lesion of mild squamous dysplasia, flanked on either side by respiratory mucosa. |
Mild dysplasia concerns changes confined to the basal third of the squamous epithelium. The epithelium itself may be mildly thickened, but maturation is complete with superficial flattening of cells above a clear prickle cell zone (Fig. 26-6). The basal cell layer may be expanded but only within the lower third of the epithelium. In this zone, there may be minimal alteration of cell size and shape, nuclei are orientated vertically, and nuclear chromatin is finely granular. Both nucleoli and mitoses are inconspicuous.
Moderate dysplasia is diagnosed when there is less subtle cytological change, which extends into the middle third of the epithelium. This thicker epithelium still shows maturation but superficial flattening of cells and prickle cells are confined to the upper third. The basal cell zone extends to involve the middle third of the epithelium where vertically orientated nuclei show still finely granular chromatin, but nuclear angulation and grooving may be appreciated. Nucleoli remain inconspicuous but mitosis may be found, confined to the lower third of the epithelium (Fig. 26-7). Cells are larger and there is some pleomorphism.
 FIGURE 26-6 High-power image of mild squamous dysplasia. There is minimal nuclear irregularity of the basal cells. |
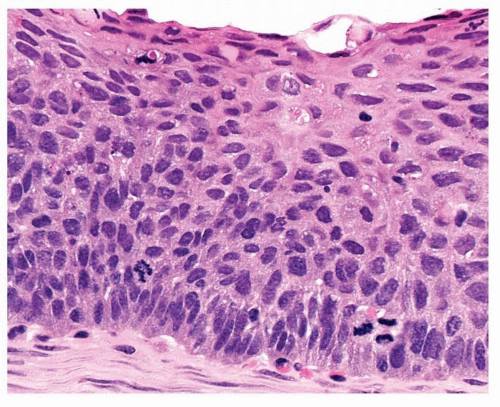 FIGURE 26-7 This area of moderate dysplasia shows basal third mitoses, more pleomorphism than in Figure 26-6, but clear evidence of superficial third maturation. |
Severe dysplasia shows atypia extending throughout the epithelial layers (Fig. 26-8). The epithelium may be notably thickened. Maturation is minimal but the most superficial cell layers may be flattened and prickle cells are rarely identified. The basal cell zone of cells with vertically orientated nuclei extends well into the upper third of the epithelium. There is now marked increase in cell size and pleomorphism. Nuclei have coarse, uneven chromatin; nuclear angulation and grooving are regularly seen. Nucleoli are prominent and mitoses may be encountered in the lower and middle thirds of the epithelium.
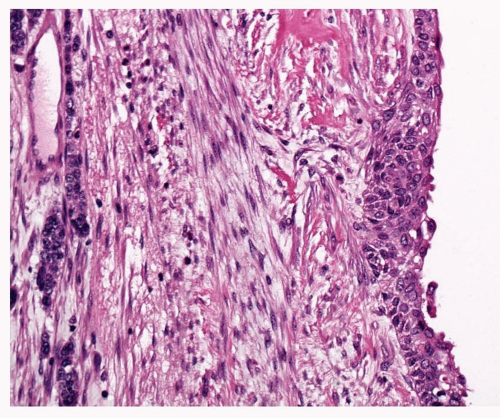
Carcinoma in situ is identified when there is marked cytological atypia and complete loss of maturation, such that if this chaotic epithelium were inverted, it would look much the same (Fig. 26-9). CIS lesions may be markedly thickened but, paradoxically, may also comprise a rather thin epithelium, making assessment difficult. Papillary architecture has been described in CIS, but caution should be taken before making a diagnosis of CIS in this situation. The WHO classification regards all papillary lesions as invasive. In CIS, mitotic figures may be found throughout the epithelium.
As the degree of atypia increases so may the thickness of the basement membrane. In severe SD and CIS, the basement membrane may become irregular, or even appear absent, but this is not
a reliable indicator of invasion. The vascularity of the stroma deep to SD/CIS may also increase. This has been regarded by some as a sign of impending invasion. A notable pattern of vascularity is seen in so-called angiogenic squamous dysplasia (ASD). In this lesion, tufts of capillary vessels protrude into the overlying squamous epithelium, which may be variably atypical (Fig. 26-10). The capillaries are usually surrounded by prominent hyaline basement membrane-like material and the epithelium over the apex of the vascular tuft may be very thin.15 The lesions may result in localized protruberance of the epithelium, giving it a papillomatous appearance; this lesion has also been referred to as micropapillomatosis (Fig. 26-11).16 ASD shows variable atypia that can be very difficult to grade according to the WHO scheme, and similar changes may be observed in nonatypical pseudostratified respiratory epithelium. ASD appears to be associated with smoking and does show molecular features consistent with neoplasia and angiogenesis.
a reliable indicator of invasion. The vascularity of the stroma deep to SD/CIS may also increase. This has been regarded by some as a sign of impending invasion. A notable pattern of vascularity is seen in so-called angiogenic squamous dysplasia (ASD). In this lesion, tufts of capillary vessels protrude into the overlying squamous epithelium, which may be variably atypical (Fig. 26-10). The capillaries are usually surrounded by prominent hyaline basement membrane-like material and the epithelium over the apex of the vascular tuft may be very thin.15 The lesions may result in localized protruberance of the epithelium, giving it a papillomatous appearance; this lesion has also been referred to as micropapillomatosis (Fig. 26-11).16 ASD shows variable atypia that can be very difficult to grade according to the WHO scheme, and similar changes may be observed in nonatypical pseudostratified respiratory epithelium. ASD appears to be associated with smoking and does show molecular features consistent with neoplasia and angiogenesis.
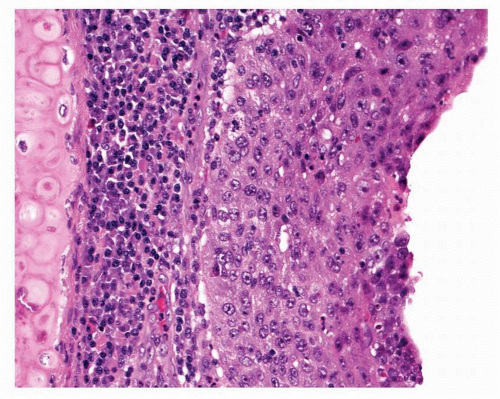 FIGURE 26-9 Squamous cell carcinoma in situ shows no maturation and marked pleomorphism. Mitotic figures may occur anywhere in the epithelium.
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|