Breast Disease: Introduction
The breast, or mammary gland, is a fibrofatty organ that produces all the necessary nutrients for a newborn. In women of childbearing age, the breast responds to cyclic hormone production and contains an abundance of epithelial structures and stroma that enable the production of milk. In postmenopausal women, declining ovarian function in late menopause leads to regression of these structures. The postmenopausal breast contains a ductal system, but the lobules shrink and collapse, leaving an organ that is composed primarily of fat. While a breast lump in a premenopausal woman is likely to be a benign problem related to cyclic hormonal changes, in a postmenopausal woman, this is not the case and the most important breast disease is cancer.
Cancer is the leading cause of death in women aged 55 to 74 years and is second to heart disease in women aged 75 and older. The incidence of cancer increases dramatically with age. In particular, breast cancer—the most common cancer in American women—is a major health concern. According to 2007 American Cancer Society estimates, breast cancer is the most common cancer in women, accounting for 31% of all newly diagnosed malignancies (178 480 new cases) and the second leading cause of cancer-related death (40 460 deaths). Moreover, U.S. incidence and mortality data from the 1980s suggest that 12% of all women will be diagnosed with breast cancer during their lifetime and that 3.5% will die from it.
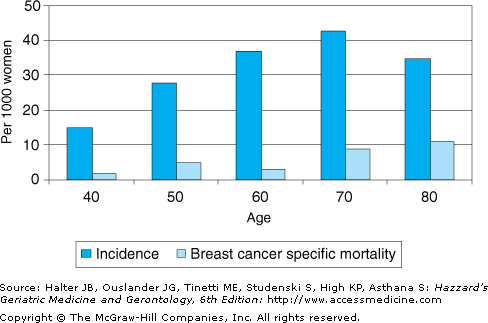
Approximately half of the cases of breast cancer occur in women older than age 65 years. In addition, 1999 National Vital Statistics indicate that the incidence increases dramatically with age (Figure 95-1) from an invasive breast cancer rate of 15 per 1000 in women aged 40 to 50 years to a rate of 43 per 1000 in women aged 70 to 80 years. Older age has also been associated with a lower breast cancer specific survival rate (Figure 95-1).
Breast cancer is a major health concern and will become of even greater importance as the size of the older population grows. Although breast cancer is more common in older women, they are also less likely to be appropriately screened, more likely to present for care at a more advanced stage and to receive inferior surgical and postoperative management, and are less likely to be entered into clinical trials.
Risk Factors and Biology
The specific cause of breast cancer is unknown. Many factors associated with increased risk have been identified. These include the following: increasing age, white race, family history of breast cancer (especially in a first-degree relative), early menarche, late age at birth of first child (older than 30 years), late menopause, history of benign breast disease (hyperplasia or atypical hyperplasia), heavy radiation exposure, obesity, increasing height, postmenopausal estrogen replacement therapy, and moderate to excessive alcohol use. Most of these factors are associated with relative risks in the range of 1.4 to 2 times the risk in the general population. There is a breast cancer risk assessment tool, based on the Gail Model, available. This tool calculates the 5-year risk of breast cancer, using risk factors of age, age at first menses, age at first live birth, presence of first-degree relatives with breast cancer, number of past breast biopsies and whether or not atypical hyperplasia was found, and race/ethnicity. There is also geographic, economic, and racial variability in breast cancer incidence, with the highest incidence being found in affluent white populations. The reason for these differences is probably related to risk factor distribution, as well as genetic and environmental factors. Although breast cancer incidence differs among racial groups, such differences are minimized or lost when racial groups that are at low risk migrate to a high-risk environment.
A family history of breast cancer, implying a genetic defect, may be important in 5% to 20% of all cases of breast cancer. Genetic predisposition is particularly important in early-onset breast cancer (diagnosed before age 50 years), but is probably not a major factor in the geriatric population. BRCA1 accounts for approximately 45% of familial breast cancers. Another gene, BRCA2, is responsible for approximately 35% of genetic abnormalities in high-risk families. Both BRCA1 and BRCA2 portend increased risks for breast cancer and ovarian cancer, ranging from 36% to 85% for breast cancer and 16% to 60% for ovarian cancer. There are many modifying factors, including genetic, hormonal, and environmental, that determine whether a genetic mutation will lead to cancer.
Most breast cancers originate from ductal epithelium. Comparisons of older and younger patients with breast cancer reveal that infiltrating ductal carcinoma is the most common histological type in both groups, accounting for 75% to 80% of cases, with lobular carcinoma accounting for approximately 5% to 10%. Mucinous carcinoma and papillary carcinoma, histological types that are associated with a somewhat lower risk of recurrence, are more common in older patients, while inflammatory carcinoma, an aggressive lesion with a poor prognosis, is extremely uncommon in such persons. Cancers in older patients are more likely to be well-differentiated and moderately differentiated, be estrogen and progesterone receptor positive (60–70% of patients), have lower rates of tumor proliferation (the number of cells synthesizing deoxyribonucleic acid [DNA]), and less frequently express the HER2 oncogene, when compared with cancers in younger patients. These data suggest that breast cancer in older patients is biologically less aggressive than it is in younger women. Mortality from breast cancer, however, is not lower in older women, leading us to explore issues associated with treatment choice and other patient- and tumor-related factors that might affect disease specific survival.
Diagnosis
Except for draconian measures such as prophylactic mastectomy, which is not 100% effective, there is no known effective means of preventing breast cancer. As the pathogenesis of breast cancer is likely to be related to interactions of estrogens, other hormones, and breast tissue, current research efforts toward prevention focus on the use of agents that lower breast tissue estrogen exposure, such as selective estrogen receptor modulators (SERMs) and aromatase inhibitors.
The SERMs, tamoxifen and raloxifene, decrease incidence of hormone receptor–positive breast cancer. Data from the Early Breast Cancer Trialists’ Collaborative Group meta-analysis involving 75 000 patients with early-stage breast cancer suggest that the use of tamoxifen decreases contralateral breast cancer risk by 40% to 50% in postmenopausal women. A national trial (National Surgical Adjuvant Breast Project [NSABP] P1) comparing tamoxifen with placebo as a means of prevention in women at high risk for breast cancer (a risk of 1.66% of invasive breast cancer over 5 years) showed that tamoxifen decreased the incidence of noninvasive and invasive breast cancer by approximately 40%, with the majority of the benefit in decreasing the risk of hormone receptor–positive breast cancer and no decrease in risk of hormone receptor–negative breast cancer. Thirty percent of women in this trial were older than 60 years and 6% were older than 70 years; similar risk reductions were seen in all age groups. A second, large randomized trial proved that raloxifene was as effective as tamoxifen in decreasing the incidence of hormone receptor–positive breast cancer. The SERMs, however, are also weakly estrogenic and increase the risk of thromboembolism. Further, tamoxifen, but not raloxifene, increases the risk of endometrial cancer. In clinical practice, the benefit of SERM treatment must be weighed against risk and can be estimated from a model developed from Gail and colleagues. At present there are large trials studying the use of aromatase inhibitors, which do not increase thromboembolic risk, compared to SERMs, to decrease the risk of breast cancer in postmenopausal women; older patients should be encouraged to participate in these trials. Maintaining an ideal body mass and exercise should also be encouraged.
Breast cancer screening in a postmenopausal woman includes mammography and a physical examination. After menopause, as estrogen levels diminish, breast glandular tissue and ductal tissue decrease and fat tissue increases. In addition, postmenopausal patients have fewer cysts and fibroadenomas. These age-related biological changes in breast tissue, especially the increased percentage of fat tissue, allow for improved contrast between small foci of malignancy and the surrounding breast tissue, resulting in fewer false-negative mammographic examinations. Several studies show that the routine use of screening mammography in women aged 50 through 70 years improves survival by detecting breast carcinoma at an earlier stage and before metastatic dissemination.
It is estimated that a 25% to 30% reduction in 5-year breast cancer mortality could be achieved if all women aged 50 to 70 years received appropriate screening with mammography. Controversy persists, however, because data are sparse in women older than 70 years. An overview analysis did suggest that mammography will be of benefit in this group. The average life expectancy of a healthy older woman who is 75 years old is 10 more years, and for a healthy woman aged 85 years, it is 7 more years, making it important to decrease cancer-related morbidity. When the estimated life expectancy exceeds 5 years, it is prudent to offer yearly screening mammogram.
Ten to twenty percent of all breast cancers are not visualized on a mammogram. Physical examination by health professionals and breast self-examination, therefore, remain essential and complementary adjuncts to mammographic screening. The patient–physician interaction is also important because, as several studies show, the most important factor for the physician to inducing an older patient to have a screening mammogram is to personally recommend it to the patient. Medicare law provides for payment for screening mammography on an annual basis. Table 95-1 presents our recommendations for the screening of older women.
FREQUENCY | COMMENT | |
|---|---|---|
Breast self-examination | Monthly | Value uncertain, but many breast cancers are still detected by the patient |
Physical examination | Yearly | By physician or other health professional; detects 15% of cancers not discovered on screening mammogram |
Mammography | Every 1–2 yr | Value in improving survival in women older than 75 yr is unproven, but extrapolation of data from studies in postmenopausal patients suggests benefit if life expectancy is 5 yr or more |
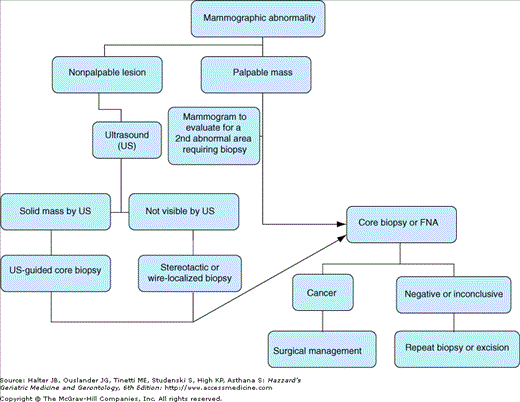
Breast cancer usually presents either as a breast mass or as a suspicious finding on a mammogram. The discovery of a breast mass in a postmenopausal woman requires prompt attention, as the majority of palpable masses in this age group are malignant. All breast masses require biopsy in this patient population. Although it is not essential to the diagnosis in a woman who presents with a mass, mammography helps to define the nature of a mass and finds other nonpalpable lesions; it is, therefore, indicated when a breast mass is found. New mammographic findings also prompt evaluation. Typically, a step-wise process of diagnosis and definitive surgery is followed, as depicted in Figure 95-2. This allows pathological confirmation of cancer and time to contemplate surgical treatment choice.
For palpable lesions, core-needle biopsy is preferable to fine-needle aspiration biopsy, because core biopsy can distinguish invasive from in situ lesions in most patients. Although concerns have been expressed that needle biopsy may be associated with tracking of malignant cells and a higher risk of local recurrence, such fears are unfounded. If the core biopsy or fine-needle aspiration is negative or inconclusive, unless the mass proves to be a cyst and resolves after aspiration, further biopsy, preferably excision, is necessary. For patients who have a mass that is characteristic of malignancy on physical examination or mammography, initial excision of the lesion or intraoperative biopsy to confirm invasive cancer and then lumpectomy or mastectomy, with or without sentinel lymph node biopsy or axillary dissection, may be preferable to a two-stage procedure involving a needle, core, or excisional biopsy.
For nonpalpable lesions that are found mammographically, ultrasound is recommended for further evaluation. If ultrasound finds a solid, suspicious lesion, ultrasound-guided biopsy can be done. If not visible by ultrasound, either stereotactic biopsy or needle-localized biopsy will be necessary. Stereotactic biopsy uses mammographic guidance to obtain a core biopsy specimen and is preferred because it allows pathologic diagnosis without anesthesia. The prone position required for the stereotactic procedure, however, may be difficult for some older patients. Needle-localized biopsy uses mammographic guidance to place a needle within the area of suspicion, after which the area is surgically excised, a procedure that typically involves anesthesia. If atypical hyperplasia is found on core biopsy, excisional biopsy is required, because a small proportion of suspicious lesions for which core biopsy finds only atypical hyperplasia will also contain in situ or invasive cancer when excised.
When biopsy is diagnostic of malignancy or definitive surgery is scheduled, preoperative evaluation includes a complete history and physical examination, a complete blood count and chemistry profile, and a chest x-ray. MRI of the breasts may also be helpful in management of older women, but is not recommended for routine screening. These studies are generally more helpful in determining the presence of comorbid illness than they are in finding metastases. Bone, computerized tomographic, or magnetic resonance imaging scans should be done to investigate signs or symptoms suggestive of metastasis, but are not necessary in asymptomatic patients. Bilateral mammography should be performed on all these patients to evaluate both the ipsilateral breast and the contralateral breast for other nonpalpable lesions.
It is important to remember that mammography is normal in 20% of patients with cancer. A palpable lesion in a postmenopausal woman always requires biopsy; mammography is of value mainly for detecting nonpalpable lesions in either the involved or the contralateral breast.
The American Joint Committee for Cancer (AJCC) guidelines set the standard for breast cancer staging. This system categorizes the extent of malignancy according to the size of the primary lesion (T), the extent of nodal involvement (N), and the occurrence of metastasis (M). Table 95-2 presents the AJCC breast cancer staging criteria. Tumor size (the largest diameter of the infiltrating component) and the extent of nodal involvement (the number of axillary nodes removed and the number positive) are determined from pathologic examination and are included in the pathology report. Less than 5% of the time, breast cancer presents as a diffuse infiltrating lesion and cannot be measured accurately. Nodal assessment is done either by sentinel lymph node biopsy or by axillary dissection. Sentinel lymph node mapping and sampling is preferred as it allows identification and assessment of the one to four nodes most likely to contain cancer. In an axillary dissection specimen, a sample of at least six nodes should be removed to reflect the prognosis accurately.
SYMBOL TNM SYSTEM | MEANING |
|---|---|
Primary tumor (T) | |
TX | Primary tumor cannot be assessed |
T0 | No evidence of primary tumor |
Tis | Carcinoma in situ; intraductal carcinoma, lobular carcinoma in situ, or Paget disease of the nipple with no tumor |
T1 | Tumor ≤ 2 cm in greatest dimension T1a ≤ 0.5 cm in greatest dimension T1b > 0.5 cm but not > 1 cm in greatest dimension T1c > 1 cm but not > 2 cm in greatest dimension |
T2 | Tumor > 2 cm but not > 5 cm in greatest dimension |
T3 | Tumor > 5 cm in greatest dimension |
T4 | Tumor of any size with direct extension to chest wall or skin (includes inflammatory carcinoma) |
Regional lymph nodes (N) | |
NX | Regional lymph nodes cannot be assessed (e.g., previously removed) |
N0 | No regional lymph-node metastasis; no additional examination for isolated tumor cells (ITCs, defined as single tumor cells or small clusters not greater than 0.2 mm, usually detected only by immunohistochemical or molecular methods but which may be verified on hematoxylin and eosin [H & E] stains). ITCs do not usually show evidence of malignant activity (e.g., proliferation or stromal reaction)
|
N1 | Metastasis in 1–3 ipsilateral axillary lymph node(s) and/or in internal mammary nodes with microscopic disease detected by SLND but not clinically apparent
|
N2 | Metastasis in 4–9 axillary lymph nodes or in clinically apparent internal mammary lymph nodes in the absence of axillary lymph nodes
|
N3 | Metastasis in 10 or more axillary lymph nodes, or in infraclavicular lymph nodes, or in clinically apparent ipsilateral internal mammary lymph nodes in the presence of one or more positive axillary nodes; or in more than three axillary lymph nodes with clinically negative microscopic metastasis in internal mammary lymph nodes; or in ipsilateral supraclavicular lymph node(s)
|
Distant metastasis (M) | |
MX | Presence of distant metastasis cannot be assessed |
M0 | No evidence of distant metastasis |
M1 | Distant metastasis (including metastasis to ipsilateral supraclavicular lymph nodes) |
Clinical stage | |
0 | Tis, N0, M0 |
1 | T1, N0, M0 |
IIA | T0, N1, M0 |
T1, N1, M0 | |
T2, N0, M0 | |
IIB | T2, N1, M0 |
T3, N0, M0 | |
IIIA | T0 or T1, N2, M0 |
T2, N2, M0 | |
T3, N1 or N2, M0 | |
IIIB | T4, any N, M0 |
Any T, N3, M0 | |
IV | Any T, any N, MI |
Other key prognostic information from the pathology report includes the following: histologic type and tumor grade, assessment of vascular or lymphatic invasion and skin involvement, and percentages of ductal carcinoma in situ and invasive carcinoma in the primary lesion. Paraffin-embedded tumor is then analyzed for other prognostic markers. Immunohistochemical analysis of the invasive cancer cells is also done to determine estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor-2 (HER2) status. An intermediate result for HER2 by immunohistochemistry necessitates analysis by fluorescent in situ hybridization (“FISH”). Other markers, such as epidermal growth factor receptor and proliferative indices, are not of consistent benefit and are not needed on a routine basis.
After definitive surgical management, asymptomatic patients who have had an unremarkable preoperative evaluation, including history and physical examination, mammography, chest x-ray, complete blood count, and serum chemistries (with liver function tests and calcium), require no further staging procedures. The use of tumor markers, such as the carcinoembryonic antigen and mucin antigens (CA27.29 and CA15.3), in patient management is controversial and is not recommended on a routine basis.
Treatment of Breast Cancer
The more widespread use of screening mammography has led to a major increase in the diagnosis of ductal carcinoma in situ (DCIS). Before the common use of screening mammograms, DCIS was uncommon, usually detected on physical examination, was large in size and the cure rate exceeded 95% with mastectomy. In a screened population, most DCIS lesions are nonpalpable and small and are suggested by microcalcifications found on screening mammography. Mastectomy cures almost all patients, but breast conserving procedures followed by breast irradiation are as effective for patients with smaller lesions.
Axillary dissection or sentinel node biopsy finds metastases in less than 1% of patients and is generally not recommended. Excision alone, without local radiation therapy, may be appropriate for patients with lesions less than 2.5 cm with generous (>1 cm) margins of normal tissue surrounding the in situ component.
Lobular carcinoma in situ (LCIS) is more common in premenopausal patients, lacks clinical and mammographic signs, is bilateral in 25% to 35% of cases, and is usually an incidental finding at breast biopsy. It is not a palpable lesion. Treatment options range from observation to bilateral mastectomy. Of note, 20% to 40% of patients with LCIS subsequently develop infiltrating ductal cancer, with both the ipsilateral breast and the contralateral breast at similar risk. LCIS serves as a high-risk marker for subsequent invasive cancer, and treatment selection must rest on the desires of the patient. Most experts recommend close follow-up of these patients, without aggressive surgery.
Once a diagnosis of breast cancer is made, a woman is counseled about surgical choices, radiation therapy, and systemic therapy. With regard to local therapy, breast-conserving procedures result in virtually identical relapse-free and overall survival compared with more extensive surgical treatment, including simple, modified-radical, and radical mastectomy. Reasonably healthy older women tolerate simple or modified mastectomies as well as younger women do, but should be offered the option of breast-conserving surgical procedures when clinically appropriate.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree