4 Brain Tumor Stem Cells
 The Cancer Stem Cell Hypothesis
The Cancer Stem Cell Hypothesis
Cancer tissues are recognized to be heterogeneous, composed of cells with different morphologies and expressing different markers. Evidence accumulated in the last 50 years suggests that intratumoral morphological heterogeneity is accompanied by functional heterogeneity; that is, not every cancer cell carries the same tumor-propagating potential.
Functional differences between cancer cells are not immediately evident on the pathology examination of specimens, but they become apparent under specific experimental settings. Experiments performed in the 1950s and 1960s, and not ethically performable today, showed that cancer cells could be transplanted subcutaneously in human subjects to generate nodules, although high numbers of cells were required for successful engraftment.1,2 These studies suggested, but did not prove, that cancers might be hierarchically organized, with only a small fraction of cells in a tumor being required for tumor growth and maintenance, hence the high number of cells required for transplantation. It was not until 1997 that a functional hierarchy was definitively and experimentally proven to exist in leukemia.3
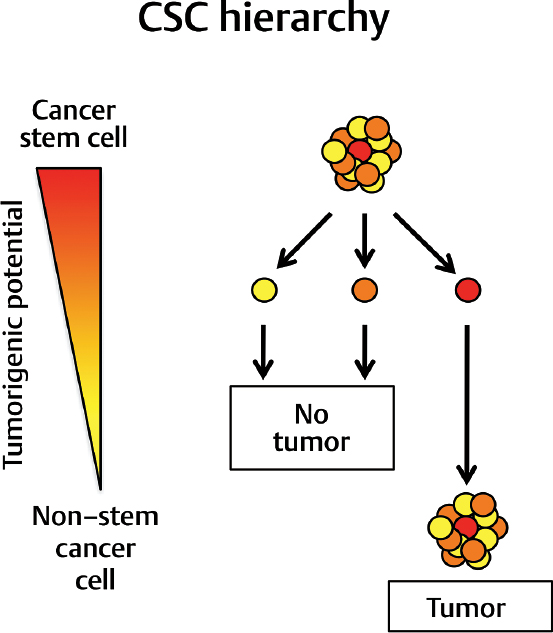
The discovery in leukemia was rapidly followed by similar findings in breast cancer4 and in brain tumors.5–9 The key findings of these studies consisted in the identification of a small population of cells characterized by defined cell surface markers in human tumors that can be experimentally transplanted orthotopically in immunocompromised mouse recipients to initiate tumor formation. Histological evaluation of the resulting tumor xenografts showed that the transplants recapitulated the main characteristics of the patients’ tumors from which they were isolated. In other words, transplantation of a small number of phenotypically defined cells into recipients was enough to generate a tumor that reproduced the entirety of the cellular heterogeneity found in the initial human specimen. These results support a “cancer stem cell” (CSC) hypothesis to explain tumor growth, by showing that tumors are organized hierarchically; not all cells in a tumor can transplant the diseases in recipients, but the ones that do produce all the cell types observed in a tumor (Fig. 4.1).
This hierarchical organization of tumor cells is reminiscent of the hierarchical structure of normal tissues. In most adult tissues, somatic stem cells maintain homeostasis by replenishing damaged or aged cells with new ones. This process requires stem cells to have the following properties: (1) selfrenewal, because they need to be able to generate more stem cells to guarantee lifelong tissue homeostasis; (2) generation of progenitor cells with extensive proliferative potential; and (3) multi-lineage differentiation, in order to provide a supply of all cell types present in a given tissue that are required for tissue functional integrity.
The term cancer stem cell derives from the biological similarity between tumors and normal tissues, in that both require a small number of cells that can self-renew and generate all the cell types observed in situ. The definition of a cancer stem cell does not require that it be derived from a normal somatic cell, although this is the case in leukemia and probably in other tumors. As the use of the term cancer stem cell has generated some confusion because it is seen as suggestive of a cell of origin for tumors, the alternative term tumorinitiating cell (TIC) has been used to provide a less phylogenetically biased and more functionally accurate definition of the cellular population residing at the top of the tumor hierarchy.
It is important to point out that the CSC hypothesis is not the only model for the organization of tumors. Another theory, the stochastic model, postulates that any cell in a tumor can acquire tumor-initiating functions, without the need for a stable functional hierarchy within the tumor.10 In light of these competing models, experimental evidence suggests that some malignancies might be organized hierarchically according to a CSC model, whereas others seem to be more in line with a stochastic model. For example, primary malignant brain tumors6,7 and acute myeloid leukemia3 appear to follow the CSC model. In contrast, B-cell lymphoblastic leukemia11 and melanoma12 appear to follow a stochastic model. Execution of in vivo transplantation experiments of primary tumor cells will be fundamental in determining the functional organization of different cancers. The key challenge to the concept of a hierarchical cellular organization in human brain tumors is that it sheds light on therapeutic failure and then leads to the development of more durable and even curative treatments (Fig. 4.2).
 Evidence for Cancer Stem Cells in Brain Tumors
Evidence for Cancer Stem Cells in Brain Tumors
Glioblastoma
Cells in brain tumors are morphologically heterogeneous, and they are known to express different markers. In the case of glioblastoma multiforme (GBM; World Health Organization [WHO] grade IV), the most aggressive and lethal adult brain tumor, the level of phenotypic heterogeneity is so noticeable that it is highlighted in the name of the disease itself. Recent evidence suggests that GBM is functionally organized as a hierarchy.
Controversy
• GBM CSCs may contribute to tumor vasculature by transdifferentiation.
Research in the early 2000s showed that cells isolated from GBM specimens based on expression of the cell surface marker CD133 (CD133+ cells) are enriched for tumorigenic potential.5–7 Notably, researchers showed that orthotopic injections of as few as 100 patient-derived CD133+ cells in immunocompromised mice resulted in the formation of GBMs that could be serially transplanted in recipients. Serial transplantation is a functional assay for long-term self-renewal potential. In contrast, transplantation of over 100,000 CD133–cells did not generate any tumors in mouse recipients.6
More recently, another group identified integrin α6 as a putative cancer stem cell marker in GBM.13 Integrin α6 is the receptor for the extracellular matrix protein laminin, and therefore it serves to illustrate a potentially important interaction between the cancer stem cells and their cellular milieu or niche. In normal development and tissue homeostasis, the stem cell niche, or microenvironment, plays an important role in regulating the self-renewal and differentiation properties of stem cells. Analogously, it is possible that the brain tumor niche affects the tumor-initiating properties of GBM stem cells. It is important to point out that the markers mentioned above represent tools to enrich for CSCs, but by no means do they capture the full heterogeneity of CSCs. Ultimately, CSCs are identified by determining their in vivo tumorigenic potential.
Another interesting function of GBM CSCs is their putative role in contributing to tumor vasculature. It was known for some time that GBMs are hypervascularized, probably in response to the high metabolic needs of a growing tumor. Furthermore, GBM CSCs may induce angiogenesis by secreting high levels of vascular endothelial growth factor (VEGF).14
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree