Brachytherapy
INTRODUCTION
Brachytherapy plays a critical role in the definitive management of primary and recurrent tumors within the uterus and vagina. As a general principle, to deliver a boost dose to gross disease, brachytherapy is preferred over external beam radiation therapy (RT) whenever brachytherapy sources can be positioned closer to the tumor than to adjacent critical structures. With brachytherapy, the physical proximity of the source to the tumor makes it possible to take advantage of the inverse square law to create a steep dose gradient and thereby deliver much high doses to tumor than to adjacent critical structures. The advantages of brachytherapy over external beam RT include the following:
Delivery of a higher mean dose. The steep dose gradient with brachytherapy means that it is often possible to deliver a higher dose to the tumor with brachytherapy than with external beam RT with the same normal tissue doses.
Reduced impact of internal organ motion. In external beam treatment planning, an additional margin is added beyond the target to account for variation in setup accuracy and internal organ motion. Because the relationship between the brachytherapy applicator and the tumor is fixed, there is no need for this additional margin in brachytherapy treatment planning.
Acceleration of treatment. Brachytherapy dose is typically given more rapidly than external beam treatment dose, reducing the opportunity for tumor cell repopulation.
Increased dose heterogeneity within the tumor. Within the target volume, brachytherapy produces significant dose heterogeneity. The potential benefits of delivering a very high dose to a portion of the tumor have not been proven; however, this heterogeneity may offer biologic advantages through immunologic mechanisms, such as enhanced tumor antigen presentation.
In addition to these general advantages over external beam RT, brachytherapy for gynecologic cancers may offer several additional advantages. First, applicator placement for treatment of most gynecologic cancers does not require a major surgical intervention. Second, the presence of a central canal from the vagina through the uterus provides a natural cavity into which applicators can be placed. Finally, although functioning of the gynecologic organs is critical for childbearing, it is not critical for patient survival; thus, it is possible to deliver ablative doses of radiation to gynecologic organs. For all of these reasons, brachytherapy has a central role in the management of gynecologic cancers.
Brachytherapy also has a number of disadvantages or challenges. These include the following:
Need for technical expertise. Brachytherapy requires a unique set of skills on the part of the physician, the dosimetrist, and the physicist. In particular, the placement of applicators in relationship to the tumor is critical and can be challenging in many cases. This is particularly true for interstitial brachytherapy.
Anesthesia and procedure risks. There are risks of anesthesia and injury from applicator placement. However, these risks are generally very small and should rarely be a reason not to perform brachytherapy.
Capital costs. Building a facility to perform brachytherapy and maintaining sources and applicators requires significant resources, necessitating a sufficient volume of patients to support a brachytherapy program.
Patient discomfort. Discomfort from applicators can be a part of the patient’s experience and requires a plan for management.
Specific indications for brachytherapy are discussed in detail in Chapters 10, 11, and 13. This chapter focuses on some general principles of gynecologic brachytherapy.
ESSENTIAL ELEMENTS OF A BRACHYTHERAPY TREATMENT PLAN
If brachytherapy is being considered as a possible treatment, it is critically important to begin preparations for brachytherapy as soon as the patient’s initial evaluation is complete. Factors that need to be considered at initial presentation include the timing of brachytherapy, applicator types that may be needed, brachytherapy placement approach, delivery method, dose rate, and fractionation.
Many aspects of the brachytherapy treatment plan are governed by the initial extent of disease. Thus, the physician who will be performing brachytherapy should always be given an opportunity to assess the patient before any treatment (including chemotherapy or external beam irradiation) has been started. Ensuring up front that all the necessary expertise and supplies are available to perform appropriate brachytherapy prevents last-minute referral to a brachytherapist. Urgent referrals can leave the brachytherapist in the difficult position of having to glean information about the initial extent of disease from review of the patient record. Furthermore, urgent referrals often result in an undesirable protraction of treatment due to delays in establishing care with a new physician at a new facility.
High-quality brachytherapy requires a coordinated team approach. The necessary steps and resources are detailed in the American Brachytherapy Society Consensus Guidelines for Locally Advanced Carcinoma of the Cervix. Part I: General Principles.1 Physicians who regularly perform brachytherapy usually work with a dedicated team of physicians, dosimetrists, and physicists using a set of established guidelines and procedures for gynecologic brachytherapy. For physicians who perform brachytherapy only occasionally, it is particularly important to explicitly verify the plan and procedures with all members of the team during each step of the procedure. Essential elements of a brachytherapy treatment plan include the following:
Anesthesia. Although some practitioners give patients only oral anxiolytics and narcotics before intrauterine applicator placement, patient discomfort with this approach can be significant and may result in suboptimal applicator placement. Anesthesia can be obtained using conscious sedation, spinal or epidural block, or general anesthesia. In our practice, we use general anesthesia for most patients who do not have a high risk for complications from anesthesia due to medical comorbidities.
Timing. It is recommended that all radiation treatment, including brachytherapy, be completed within 56 days. Brachytherapy should be started no later than 6 weeks after the start of pelvic RT. For patients with cervical cancer, the first implant can be performed after completion of external beam RT or earlier if the disease is small or has responded sufficiently to allow for good tumor coverage (e.g., if a central tumor has shrunk to <4 cm). Any nodal or parametrial boosts should be interdigitated between brachytherapy treatments. Prolonged treatment results in lower rates of local control and survival (Chapter 7).2
Applicator selection. It is critical to select the best applicators for each patient’s anatomy. The features of various gynecologic brachytherapy applicators are discussed in more detail below.
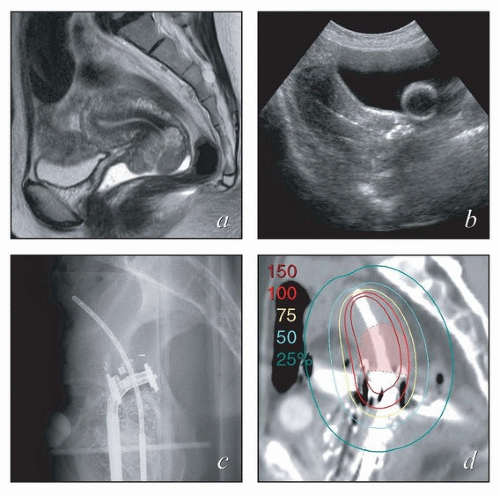
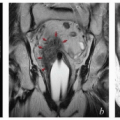
Physician expertise. Optimal placement of intrauterine intracavitary applicators requires familiarity with the processes of sounding the uterus, often in the setting of extensive disease, and placing applicators within the vagina. Initial clinical and radiographic findings can help to identify cases that are likely to be particularly challenging. Challenging cases can often be identified prospectively through tumor factors, such as disease obstructing the cervical canal, and patient-specific factors, such as obesity, retroverted uterus, or narrow vagina (Fig. 8.1). For cases like these, it may be useful to arrange for a gynecologic oncologist to be present during applicator placement. Placement of interstitial needles is particularly complex and requires specialized expertise.
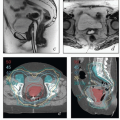
Intraoperative imaging. Intraoperative imaging is critical to ensure that the tandem is well placed in the uterus, the vaginal applicators are flush with the cervix, and adequate vaginal packing is present to reduce the dose to the bladder and rectum. The combination of transabdominal sonograms and orthogonal films can provide a great deal of information about the applicator position (Fig. 8.2). Transabdominal ultrasonography is particularly helpful to allow visualization of tandem placement in real time during the procedure. Orthogonal radiographs can be used to identify the proximity of the vaginal applicators to the cervix as marked with radiopaque markers. An alternative approach is to use intraoperative axial imaging with magnetic resonance imaging (MRI) or computed tomography (CT). The relative advantages of these imaging techniques are discussed below.
Physics expertise. Brachytherapy treatment planning is a unique discipline that requires dedicated physics support. It is important to ensure that a physicist with necessary expertise will be available for the procedure.
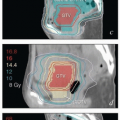
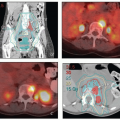
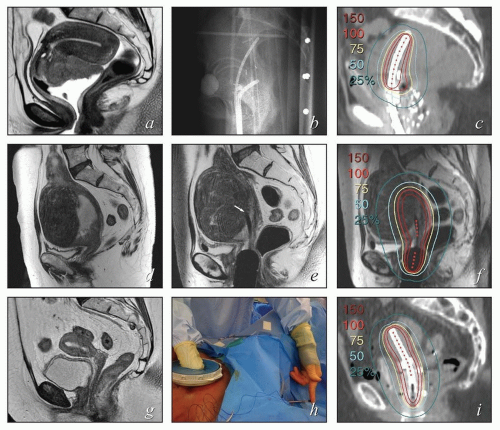 FIGURE 8.1 Initial imaging features may suggest cases that require special preparation for brachytherapy. The three patients illustrated here had rare anatomical features that were predicted to pose unusual challenges for brachytherapy. The first patient (a-c) had bulky disease involving the posterior lip of the cervix and a sharply retroflexed uterus that was essentially trapped in the sacral hollow (a). MRI performed before brachytherapy showed an excellent response, but the uterine position was unchanged and, at brachytherapy, could not be moved to a neutral position. However, an unusual brachytherapy arrangement (c) provided excellent coverage of the posterior lip of the cervix. At this writing, the patient had no evidence of disease (NED) at 3 years. The second patient (d-f) had a large uterine fibroid, with pyometra above an obstructing cervical cancer and parametrial involvement (d and Fig. 4.7). It was anticipated that the fibroid would impede optimal brachytherapy applicator placement. Arrangements were made for pelvic RT followed by MRI-guided brachytherapy; the patient was counseled that she might receive either definitive brachytherapy or a reduced dose followed by hysterectomy. At 5 weeks, MRI and examination under anesthesia showed a near complete tumor response. PDR brachytherapy was performed, but the tandem could not be passed beyond the fibroid (arrow, e). Minimal dwell times were allocated to positions close to the rectum; total HRCTV D90 and D2cc rectal doses were 73 and 68 Gy, respectively. Four weeks later (f), hysterectomy was performed. The third patient (g-i) had a narrow vagina and a tiny uterus with a small tumor that replaced the cervix and invaded the parametrium and vaginal fornix (g). After external beam RT, no cervical os was visible, and initial efforts to sound the uterus failed. Because the surgeon felt the patient was still a poor candidate for hysterectomy, a second attempt was made to sound the uterus. Using a 4 cm incision made by the gynecologic oncologist, the radiation oncologist inserted a hand into the pelvis, grasped the cervix between the thumb and forefinger, and, using the opposite (left) hand, successfully threaded the tandem through the obstructed canal (h). The patient received a single 66-hour treatment, bringing the total HRCTV D90 dose to 85 Gy, although Point A received only 63 Gy (i); the D2cc doses to bladder and rectum were 63 and 60 Gy, respectively. |
GYNECOLOGIC BRACHYTHERAPY APPLICATORS
Intravaginal Applicators
Single-Channel Vaginal Cylinders
Single-channel vaginal cylinders are useful if the surface of the entire circumference of the vagina requires treatment to a similar dose. Because of the rapid falloff in dose with increasing distance from the surface, intracavitary cylinders are not appropriate to treat tumors that are more than 4 to 5 mm thick.
Vaginal cylinders that are placed in the vagina after hysterectomy typically have a domed apical surface. These dome cylinders can be purchased as a single unit that extends along the
length of the vagina or as a dome that can be assembled with vaginal cylinders to create an applicator of the desired length.
length of the vagina or as a dome that can be assembled with vaginal cylinders to create an applicator of the desired length.
Single-channel domed vaginal cylinders are ideal for prophylactic treatment of the apical vagina in patients who are at risk for vaginal recurrence after hysterectomy for endometrial cancer (Chapter 13). In selected cases, a domed cylinder may be used to deliver a boost dose to the vaginal surface after postoperative external beam RT for cervical or endometrial cancer (e.g., CS 10.1, 13.1).
Vaginal intracavitary brachytherapy may also be used in the definitive treatment of primary or recurrent cancers that involve the upper vagina if the disease is no more than 5 mm thick at the time of brachytherapy (CS 11.1). If there is any uncertainty about the thickness of the lesion, it should be evaluated using MRI or ultrasonography before brachytherapy is performed. To accurately determine the lesion thickness, MRI should always be performed with an MR-compatible cylinder or gel in the vagina (Chapter 4).
For patients who have an intact uterus, vaginal cylinders may be placed in the vagina in conjunction with an intrauterine tandem. Cylinders are typically used in this way to treat patients with cervical cancer who have a narrow vagina or to treat areas of superficial vaginal involvement. Again, disease that is more than 5 mm thick should be treated using another method, usually interstitial brachytherapy.
Domed vaginal cylinders come in widths ranging from 1.5 to 4.5 cm to accommodate variations in vaginal capacity. For most patients with endometrial cancer, a cylinder with a diameter of 2.5 to 3.5 cm provides the best fit for posthysterectomy vaginal cuff treatment. Because of the rapid falloff of radiation dose from the surface of vaginal cylinders, the effectiveness of treatment depends on achievement of an accurate fit. Poor selection of an intracavitary applicator can lead to underdosage of the vagina in several ways:
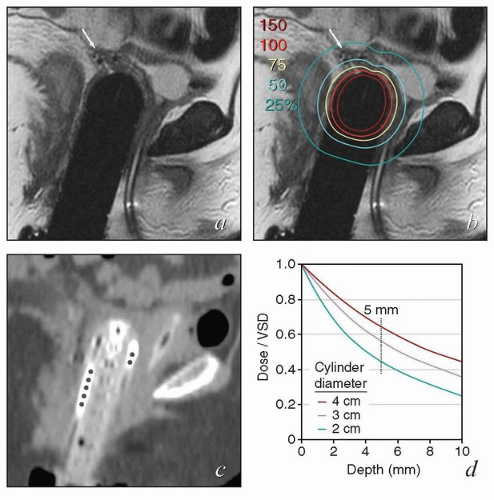
If a very small cylinder is placed in a capacious vagina, folds in the lateral vaginal mucosa will not be in contact with the cylinder and will, therefore, receive less than the prescribed dose of radiation. Failure to fully advance the dome can also cause a portion of the vagina to receive an inadequate dose (Fig. 8.3).
A domed shape is optimal for most posthysterectomy vaginal cuff treatments; however, in some cases, the surgeon sutures the vagina in a way that leaves pockets or “rabbit-ears” at the lateral aspects of the apical vaginal incision site. The bases of these pockets may be displaced a sufficient distance from the dome surface to cause significant underdosage. In some of these cases, vaginal ovoids provide a better distribution of radiation dose than can be achieved with a domed cylinder; image guidance is particularly helpful in such cases.
An overly large dome may not reach the vaginal apex. If the vagina is tapered at the apex, it is sometimes necessary to use different widths for the domed and distal cylinders, particularly if more than the apical vagina requires treatment.
Most cylindrical vaginal applicators can be purchased as stackable segments. Additional cylinders are added to the dome or are stacked distal to the stopper on an intrauterine tandem to tailor the applicator length to the length of the vagina. At a minimum, the applicator should extend at least 2 cm beyond the most distal source or active dwell position to ensure that the vagina does not collapse around active sources in the stem of the applicator, resulting in a high dose to the vaginal mucosa. Ideally, for prophylactic vaginal cuff treatment, the distal limit of the applicator
should lie just inside the introitus. For treatment of gross disease, the dome and cylinders should fill the entire vagina and extend at least 2 cm distal to the distal limit of the target volume.
should lie just inside the introitus. For treatment of gross disease, the dome and cylinders should fill the entire vagina and extend at least 2 cm distal to the distal limit of the target volume.
Fixation devices can be used to assure that the dome remains in the correct position throughout high-dose rate (HDR) treatment (Fig. 8.4). For patients receiving low-dose rate (LDR) or pulsed-dose rate (PDR) treatment, the applicator should be secured with a suture passed through the labia. Vaginal cylinders that protrude beyond the perineal ring must be secured during treatment. The position of a vaginal dome or tandem, and cylinders can shift slightly when the legs are brought down from the lithotomy position. For this reason, verification films or CT scans should always be obtained with the patient in the treatment position.
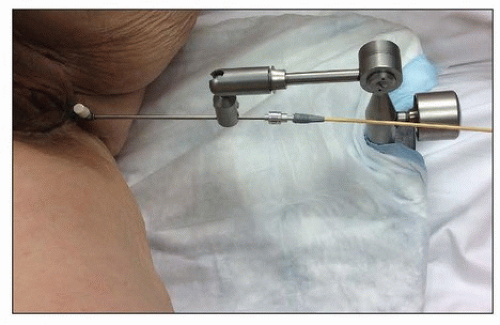 FIGURE 8.4 A fixation device used to ensure that the domed vaginal cylinder remained flush against the vaginal mucosa throughout treatment. |
Multichannel Vaginal Cylinders
Vaginal cylinders with circumferential peripheral channels allow eccentric distribution of the surface radiation dose. In most cases, these channels do not reach into the dome itself.
Multichannel cylinders typically contain 5 to 6 channels that are evenly spaced 3 to 5 mm deep to the surface of the cylinder; however, cylindrical vaginal applicators have been manufactured that contain up to 13 channels.
For prophylactic vaginal cuff irradiation, multichannel cylinders offer few clinical advantages over single-channel cylinders because the goal of treatment, that is, to achieve a homogeneous dose to at-risk apical vaginal mucosa (Chapter 13), is achieved nicely with a single-channel domed cylinder.
Multichannel vaginal cylinders can be helpful for treatment of primary or recurrent cancers that superficially involve the midvagina. Because the peripheral channels are usually <5 mm deep to the cylinder surface, the falloff in dose beyond the vaginal mucosa is very steep: typically the dose contributed from peripheral channels to tissues 5 mm deep to the applicator surface is <25% of the surface dose. Depending on the depth and separation of the channels, the surface dose may also be quite heterogeneous, with hot spots overlying the activated channels. For these reasons, it is recommended that the peripheral channels be used only as a supplement to the
dose delivered from the central channel, usually for lesions that are <3 mm thick (CS 11.2, 11.6, 13.3) or in combination with interstitial needles (CS 11.3, 11.5, 11.7).
dose delivered from the central channel, usually for lesions that are <3 mm thick (CS 11.2, 11.6, 13.3) or in combination with interstitial needles (CS 11.3, 11.5, 11.7).
Multichannel vaginal cylinders must always be secured during treatment to prevent rotation of the applicator.
Vaginal Colpostats or “Ovoids”
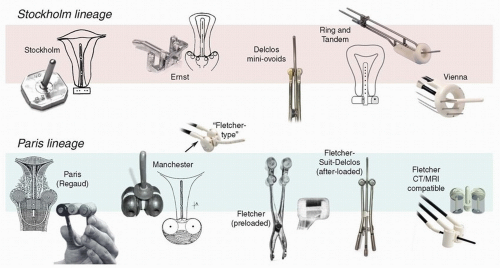
First developed in Paris in the early 20th century, colpostats, also known as “ovoids,” are paired vaginal applicators that distance intravaginal sources from the mucosa and ideally fit adjacent to the cervix in the vaginal fornices. The goal of this arrangement is to maximize the dose to lateral parametrial tissues with relatively less exposure of anterior and posterior critical structures. Subsequent innovations led to adjustments in the shape of the ovoids and in the angle and position of the source channels; the addition of internal shielding; and the development of manual and remote methods of afterloading (Fig. 8.5).3 Today, these applicators come in a wide variety of configurations. Although many are labeled “Manchester” or “Fletcher” ovoids, these frequently bear little resemblance
to the applicators that were designed in Manchester or by Dr. Fletcher, respectively. For this reason, it is always preferable to look at the individual features to determine which type of applicator is best suited to a specific clinical setting.
to the applicators that were designed in Manchester or by Dr. Fletcher, respectively. For this reason, it is always preferable to look at the individual features to determine which type of applicator is best suited to a specific clinical setting.
Key features that influence the distribution of radiation surrounding vaginal colpostats include the following:
The angle of the source channels vis à vis the intrauterine tandem. Most colpostats have been designed to position a 2-cm tube source or a line of source positions approximately perpendicular to the axis of the vagina and uterus (Fig. 8.5). This orientation takes advantage of the anisotropy of the sources and decreases the dose to rectum and bladder relative to lateral structures. In contrast, colpostats that have the source channel positioned in parallel with the vaginal axis tend to provide less sparing of critical structures. One example of such a colpostat is the Henschke applicator, an early afterloaded applicator that was originally developed at Memorial Sloan Kettering but is still available in several forms today.
The shape of the colpostat. Early colpostats, such as those designed in Manchester in the 1930s, were truly ovoid. In the 1950s, Fletcher developed a cylindrical ovoid designed to provide greater distance between the rectovaginal septum and the radiation sources and to accommodate vaginal shields; this applicator was the most popular in the United States for several decades. The Fletcher-Williamson applicator, which has all the key features of earlier shielded applicators, was developed in the 2000s for use with remote afterloading units. In recent years, there has been increased use of unshielded ovoids similar in shape to Manchester ovoids; these are somewhat easier to insert in the vagina but generally accommodate less activity than true Fletcher ovoids because of the shape, lack of shielding, and other features (Fig. 8.5).
The size of the colpostat. Most colpostats come in a variety of sizes, with a diameter ranging from 15 to 30 mm. Historically, it was recommended that clinicians use the largest ovoids that could be accommodated by the vagina because large ovoids could be loaded more heavily and achieve greater lateral penetration of dose than was possible with 2-cm ovoids. However, 25- to 30-mm ovoids also tend to expose a large vaginal surface area to a high dose of radiation. With the advent of chemoradiation, the cervix is usually small at the time of brachytherapy, and 2-cm ovoids are usually sufficient. Mini-ovoids were originally designed to be used in patients whose narrow vaginas did not accommodate 2-cm Fletcher-Suit-Delclos ovoids (Fig. 8.5). With similar source loadings, the dose at the surface of a mini-ovoid is two to three times the dose at the surface of a 2-cm Fletcher-Suit-Delclos ovoid. For this reason, and because mini-ovoids lack internal shields, mini-ovoids can safely accommodate only about half as much activity as a 2-cm Fletcher-Suit-Delclos ovoid or Fletcher-Williamson ovoid.
Internal shields. Internal shields have been a hallmark of all variations of the 2-cm Fletcher, Fletcher-Suit, and Fletcher-Suit-Delclos colpostats. Internal shields have been demonstrated to reduce the dose to the rectum and bladder by as much as 25%.4 Many of the applicators that are currently marketed as “Fletcher” or “Fletcher-type” ovoids lack internal shields and other signature features of true Fletcher colpostats.
Position of the source channel. In order to accommodate limitations on the curvature of remote-afterloading applicators, some modern ovoids have source channels that are not perfectly centered in the ovoid, with the source closer to the surface posteriorly than at the center of the ovoid. This can cause higher than expected doses to be delivered to some portions of the vaginal mucosa and rectum, particularly if image guidance is not used.
Separation of the ovoids. For any given source loading, the dose to the bladder and rectum increases markedly as the distance between the sources decreases. However, excessive separation of the ovoids creates a cold spot in the distribution of dose to the distal cervix.
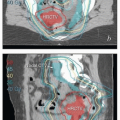
MRI and CT compatibility. Fletcher-Suit-Delclos ovoids (and Fletcher-Williamson ovoids, which were designed for remote afterloading) are not MRI compatible and cause substantial artifact on CT. At MD Anderson, we utilize a novel applicator with movable shields made of nonferrous metal.5 These shields make it possible to obtain high-quality CT and MR images without sacrificing the dosimetric benefits of ovoid shields (Fig. 8.6). Most recently, unshielded ovoids have been developed that contain a passageway through which interstitial needles can be passed through the ovoid into the cervix.6
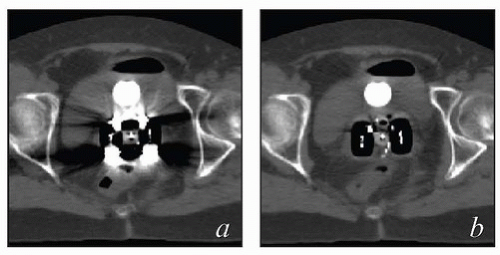 FIGURE 8.6 One disadvantage of shielded Fletcher ovoids is the CT artifact caused by the shields (Fig. 8.2d). To overcome this problem, ovoids have been developed with movable shields that can be shifted out of the image acquisition plane during CT. On the left (a) is an axial image with the shields in the treatment position throughout CT acquisition. On the right is an image taken with the shields shifted first anteriorly and then posteriorly to keep them out of the CT plane (b). The ovoids are also MRI compatible.
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|