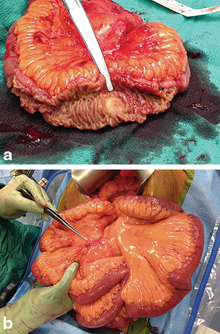
Fig. 1
(a) Intraoperative photo of small bowel obstruction due to kinking caused by neuroendocrine tumor. (b) CT scan image demonstrating the same neuroendocrine tumor (indicated by arrow)
Carcinoid Syndrome
The term carcinoid syndrome was first described in 1954 by Thorson et al., who were the first to draw a correlation between certain unusual clinical symptoms in patients with metastatic small bowel NETs . These symptoms include flushing, diarrhea, bronchospasm, and right-sided valvular heart disease [9]. Metastatic NETs of the midgut most commonly produce the classical carcinoid syndrome [1]. These manifestations are associated with the release of various substances from the tumor, including serotonin, 5-hydroxytryptophan (HTP), histamine, dopamine, vasoactive intestinal peptide, and prostaglandins [5]. The liver detoxifies the substances that are released by primary small bowel NETs and mesenteric metastases as these pass through the portal circulation into the liver. Serotonin is metabolized in the liver to 5-hydroxyindoleacetic acid (5-HIAA), a urinary metabolite. Carcinoid syndrome occurs in patients with liver metastasis in which the tumors can secrete vasoactive substances directly into the systemic circulation and bypass the portal circulation [10].
Flushing is the most common symptom of carcinoid syndrome and occurs in 73 % of patients. It presents as a diffuse erythematous flushing of the face, neck, and upper torso, which may be precipitated by exercise, stress, alcohol, or certain foods. Typically, patients experience symptoms episodically for 2–10 min. It is attributed to the release of prostaglandins, tachykinins, and serotonin from the tumors [11, 12] . Diarrhea is the next most commonly observed symptom, with approximately 65 % of patients affected. Serotonin, prostaglandins, histamines, and tachykinins are involved in the pathogenesis of the diarrhea by stimulating peristalsis and therefore resulting in increased colonic transit times. They may also affect intestinal fluid and electrolyte secretion via 5-HT2A receptors [13, 14]. Approximately 10 % of patients develop carcinoid heart disease over time due to long-standing elevations in circulating serotonin levels. Patients may develop tricuspid regurgitation and pulmonic stenosis due to deposition of fibrotic tissue on the valves [15]. The right heart is affected preferentially over the left side due to the direct exposure to vasoactive substances secreted by liver metastases, whereas the pulmonary circulation inactivates the hormones prior to exposure of the left side of the heart [16]. Patients with carcinoid heart disease may require heart surgery or valve replacement [5]. Bronchoconstriction occurs in 8 % of cases of carcinoid syndrome and is manifested as wheezing [14].
Diagnosis
Biochemical Studies
Small bowel NETs produce both functional hormones and nonfunctional proteins that are released from their secretory vesicles. These may be used as biochemical markers to aid in the diagnosis and management of patients with small bowel NETs.
Urinary 5-HIAA, a metabolite of serotonin, is useful for the diagnosis and monitoring of patients with small bowel NETs. The measurement of 5-HIAA in a 24-h urine sample collected under strict dietary restrictions has a sensitivity of 73 % for localized disease and 100 % for metastatic disease. It has a specificity of 100 % for the presence of midgut carcinoid but is less useful for foregut and hindgut NETs, as these rarely produce serotonin [5, 14]. Although normal ranges vary by laboratory, the normal range for 24-h urine 5-HIAA is between 2 and 15 mg/day. Certain drugs such as selective serotonin reuptake inhibitors, foods rich in tryptophan, and malabsorption syndromes such as celiac disease may increase 24-h urine 5-HIAA levels and produce a false positive result. Patients with carcinoid syndrome may have levels greater than 100 mg/day [1, 17].
When urinary 5-HIAA values are equivocal, whole blood serotonin levels may be helpful in making the diagnosis. The range of normal values for whole blood fasting serotonin levels varies between 71 and 310 ng/ml and can be extremely elevated in patients with carcinoid syndrome, with values as high as 790–4500 ng/ml [18]. The sensitivity and specificity of blood serotonin assays have not been well established, and false-positive results may occur due to the release of platelet serotonin [1].
Chromogranins are glycoproteins that are stored within secretory vesicles and released with peptides and amines from neuroendocrine tissues. Well-differentiated NETs release elevated levels of chromogranin [1]. Chromogranin A is the most sensitive among the subtypes (chromogranin A, B, and C), and it is a sensitive but not specific marker for NETs. The sensitivity and specificity of chromogranin A depend on the set cutoff value; a level greater than 32 U/L has a sensitivity of 75 % and specificity of 84 %. There is a correlation between chromogranin A levels and tumor burden, and a level greater than 5000 g/L is an independent predictor of poor overall survival [19]. False-positive chromogranin A levels may result from atrophic gastritis, renal dysfunction, inflammatory bowel disease, and the use of proton pump inhibitors [20, 21]. Chromogranin A is used as a tumor marker in patients with an established diagnosis of NET, rather than as a screening test due to its low specificity. Levels can be followed to monitor disease progression, response to therapy, and surveillance for recurrence [22].
Various other secretory proteins have been proposed and studied as possible useful tumor markers for small bowel NETs. These include neuron specific enolase (NSE), substance P, neurokinin A, and pancreastatin. Although some studies have found that changes in these tumor markers correlate with treatment response, tumor progression, or recurrence, chromogranin A still remains a more sensitive marker [23, 24]. The latest 2014 update of the National Comprehensive Cancer Network (NCCN) guidelines for NETs still does not recommend routine measurement of any tumor marker [25].
Imaging
Primary small bowel NETs are often small in size and therefore difficult to identify in imaging studies. Lesions are more often identified by imaging studies once there is metastatic involvement of the mesentery or the liver [5]. The primary imaging modalities used to evaluate small bowel NETs are computed tomography (CT) scan, magnetic resonance imaging (MRI), and somatostatin receptor scintigraphy (SRS). Also, several novel imaging techniques are emerging using various positron emission tomography (PET) radiotracers [1].
Small bowel NETs may produce mesenteric masses by either direct tumor involvement or metastasis to lymph nodes. These mesenteric masses are best evaluated by contrast-enhanced CT scan, which can demonstrate the dense desmoplastic fibrosis, often tethering the surrounding small bowel. Additionally, contrast enhancement aids in the evaluation of resectability by visualizing the major mesenteric vessels and their relation to the tumor (Fig. 2) [1, 5]. CT enterography also may be employed to identify primary small bowel tumors that are not visualized on standard CT scans [26]. Triple-phase CT scans are preferred when evaluating liver metastases, as NET metastatic lesions are hypervascular and therefore exhibit a pattern of enhancement during the early arterial phase and rapid washout during the portal venous phase [27].
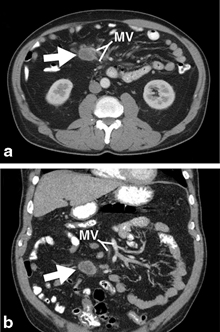

Fig. 2
Axial (a) and coronal (b) CT scan images demonstrating a neuroendocrine tumor in the mesentery (indicated by arrow) and its relationship to the mesenteric vessels ( MV)
MRI can delineate and detect liver metastases and is more sensitive than other imaging modalities. In a series of patients with metastatic small bowel NET to liver, MRI was able to detect more hepatic metastases than CT or SRS. Additionally, MRI has the ability to detect subcentimeter hepatic lesions, which is of benefit when planning cytoreductive hepatic surgery [28].
Small bowel NETs often express somatostatin receptors, which bind to the somatostatin analogue octreotide. SRS (e.g., Octreoscan) uses indium-111-labeled octreotide to identify areas of uptake in the body. This imaging modality visualizes the entire body, enabling the detection of active lesions throughout the body. The sensitivity of SRS has been reported to be greater than 90 % [1, 5]. This imaging modality also provides useful information on the somatostatin receptor status of the tumor to determine if the patient is eligible for treatment with somatostatin analogues [29]. Improvements in CT and MRI technology have led to a decline in the use of SRS in the workup of NETs. In one series, CT and MRI were superior to SRS at detecting pathologic lesions and found that SRS was inferior at detecting metastatic lesions less than 1.5 cm with a reported sensitivity of only 35 % [28].
Recently, functional PET imaging modalities have emerged as novel methods to visualize NETs. Their advantages include improved spatial resolution over CT and SRS, and improved sensitivity for detection of small lesions. The radiotracers used are not yet widely available but include 18F-dihydroxyphenylalanine (18F-DOPA), 11C-5-hydroxytrytophan (11C-5-HTP), and 68 Ga-DOTATOC. Due to the limited availability of these radiopharmaceuticals, they are generally used only when other imaging techniques are unsuccessful. Fluorodeoxyglucose (FDG)-PET scans are typically not used in the imaging workup of NETs because FDG only accumulates in high-grade NETs [29, 30]. FDG positivity is associated with early tumor progression and a higher risk of death. Recent studies have proposed the use of FDG-PET as a method to identify tumors with a higher malignant potential [31, 32].
Pathology and Staging
The clinical behavior of small bowel NETs is closely associated with the histological grade and differentiation of the tumors. Histological grade is determined by the proliferative activity of the tumor, which is measured by the mitotic rate and the Ki-67 index. Differentiation of the tumor describes how closely the tumor cells resemble normal endocrine cells, where well-differentiated tumors closely resemble normal endocrine tissues and poorly differentiated do not. The classification of NETs has evolved over the years, and in 2007 the European Neuroendocrine Tumor Society (ENETS) proposed a formal tumor, node, metastasis (TNM) staging for tumors of the lower jejunum and ileum, as well as incorporated a histologic grade into the new classification system. In 2010, the American Joint Committee on Cancer (AJCC) adopted this classification system. The TNM classification distinguishes between tumors that remain within the submucosa and are smaller than 1 cm (T1), tumors that invade muscularis propia or are larger than 1 cm (T2), tumors that invade through the muscularis propia into the subserosa or nonperitonealized tissue (T3), and tumors that invade peritoneum or other organs (T4; Table 1) [33, 34]. The World Health Organization’s pathological classification describes well-differentiated tumors as low grade (< 2 mitoses per 10 high-power field [HPF] and ≤ 2 % Ki67 index) or intermediate grade (2–10 mitoses per 10 HPF or 3–20 % Ki67 index). Poorly differentiated tumors are high grade (> 20 mitoses per 10 HPF or > 20 % Ki67 index) (Table 2) [35].
Table 1
TNM staging classification of small bowel NETs. (From [33]. Reprinted with permission from Springer)
T1 | Tumor invades lamina propia or submucosa and is 1 cm or less | ||
T2 | Tumor invades muscularis propia or is greater than 1 cm | ||
T3 | Tumor invades through the muscularis propia, into the subserosa, or into non-peritonealized tissue | ||
T4 | Tumor invades the peritoneum or any other organs or structures | ||
N0 | No regional lymph node metastasis | ||
N1 | Regional lymph node metastasis | ||
M0 | No distant metastasis | ||
M1 | Distant metastasis | ||
Stage | T | N | M |
I | T1 | N0 | M0 |
IIA | T2 | N0 | M0 |
IIB | T3 | N0 | M0 |
IIIA | T4 | N0 | M0 |
IIIB | Any T | N1 | M0 |
IV | Any T | Any N | M1 |
Differentiation | Grade | Criteria |
|---|---|---|
Well differentiated | Low (typical) | < 2 mitoses/10HPF and < 2 % Ki67 index |
Intermediate (atypical) | 2–20 mitoses/10HPF or 3–20 % Ki67 index | |
Poorly differentiated | High | > 20 mitoses/10HPF or > 20 % Ki67 index |
A recent study by Strosberg et al. examined the prognostic validity of the AJCC staging classification by looking at 691 patients with jejunal–ileal NETs and examining overall survival based on stage. The 5-year survival rate was 100 % for stage I and II tumors, 91 % for stage III tumors, and 72 % for stage IV tumors. There was a difference in survival in the stage IIIB patients based on resectability of the tumor, with a 95 % 5-year survival for resectable tumors compared to a 78 % 5-year survival for unresectable tumors [36].
Management
Surgery
Management of small bowel NETs requires a multidisciplinary approach to achieve the best long-term survival rates. Aggressive management with surgery, embolization of metastases, radiotherapy, metabolic therapy, and chemotherapy can achieve long-term survival even in patients with advanced disease. Nevertheless, surgical resection with removal of the primary tumor with regional lymph nodes and resection of metastatic lesions when possible is the only hope for cure and is only possible in about 20 % of cases [37]. Surgery for resection of the primary tumor may be necessary even in patients with unresectable metastases to treat or prevent local complications such as intestinal obstruction, ischemia, or bleeding, as well as to provide symptomatic control of carcinoid syndrome [38]. Although some controversy exits regarding whether resection of the primary tumor in these cases is associated with improved survival, most experts agree on resection for prevention of local complications from small bowel NETs in patients who are likely to survive long enough to experience symptoms [39].
The most common location for small bowel NETs is the ileum , followed by jejunum . Surgical resection is determined by the precise location of the tumor. Tumors in the distal ileum near the ileocecal valve are treated with right hemicolectomy. Small bowel NETs located more proximal in the small bowel are managed by partial small bowel resection (Fig. 3). Although multifocality is rare, the entire bowel should be inspected for additional synchronous lesions and lymphatic metastases. Regional mesenteric lymph nodes should be resected in conjunction with the small bowel in order to examine for lymph node metastases, a practice that has been associated with improved survival [39, 40]. Lymph node metastases at the root of the mesentery may involve the superior mesenteric vessels, and these cases should be referred to high-volume centers with surgical expertise. A laparoscopic approach may be used in certain cases to resect primary small bowel NETs and is associated with shorter hospital stays and decreased morbidity [37]. Radioguided exploration has recently been explored as a novel tool to assist in the cytoreductive surgery for metastatic small bowel NETs [41].