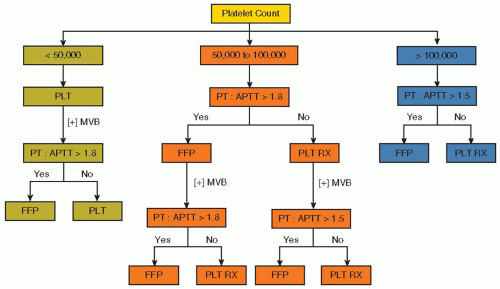
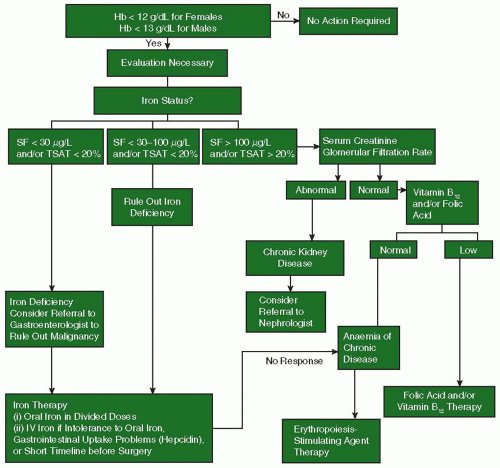
Genetic and Acquired Clotting Abnormalities
Hereditary coagulation deficiencies are usually identified by history or screening test and are confirmed by specific laboratory evaluation. The most common inherited coagulation protein deficiencies are hemophilia A and B and vWD. Patients with hemophilia A or B may develop IgG antibodies to the deficient coagulation proteins, FVIII or FIX, from chronic replacement therapy. Deficiencies in coagulation proteins are usually treated by replacement therapy, and many of these are now available as recombinant human proteins. Inherited deficiencies in fibrinolytic inhibitors, α
2-antiplasmin or plasminogen activator inhibitor-1, may also cause substantial bleeding due to excessive fibrinolysis, and these are effectively treated by lysine analogs, epsilon aminocaproic acid, or tranexamic acid.
26 A variety of genetic defects may affect platelets and disturb platelet numbers and function.
27 Some may respond to desmopressin or require platelet transfusions, but consultation from an expert hematologist is needed, particularly since patients may develop antibodies against the diminished receptors from prior transfusions. Osler-Weber-Rendu and Ehlers-Danlos syndromes may cause troublesome bleeding problems during surgery even though circulating coagulation factors and platelets are normal. Marfan syndrome is not associated with increased risk of bleeding.
27Acquired bleeding disorders of coagulation proteins, fibrinolysis, and platelets occur more often than hereditary bleeding diseases. Drug-induced antibodies (quinidine, quinine, some antibiotics) against specific coagulation proteins are uncommon. Surgeons, however, may encounter patients with antibodies to factor V due to prior use of topical bovine fibrin glue for local hemostasis.
28,
29 Advanced liver disease may diminish nearly all
coagulation and regulatory proteins of the coagulation system to levels that affect hemostasis and/or promote severe fibrinolysis.
30 However, drugs prescribed to inhibit platelet function cause the most common acquired bleeding disorders that concern surgeons.
Surgeons must also be aware of a large menu of genetic procoagulant clotting disorders that increase the likelihood of thrombotic and embolic complications. Factor V
Leiden mutation, which impairs activation of the natural anticoagulant protein C, is prevalent (2% to 5%) in Northern European whites and their descendants and the prothrombin 20,210 mutation is prevalent (1% to 3%) in Southern Europeans.
31 Both mutations increase the risk of venous thrombosis.
31 Lupus anticoagulant and anticardiolipin antibodies may produce thrombotic complications during and after major surgery.
32Platelets. Thrombocytopenia or platelet dysfunction that are not drug related occur in patients with myeloproliferative diseases, uremia, acquired vWD, antiplatelet antibodies (e.g., immune thrombocytopenia), posttransfusion purpura, and cirrhosis.
33 Aspirin and some NSAIDs irreversibly inactivate platelet cyclooxygenase for the life of the platelet (˜10 days). Cyclooxygenase blockade partially inhibits platelet function by preventing endoperoxide and thromboxane A2 synthesis.
34 Aspirin prolongs the bleeding time
35 and blocks platelet aggregation,
36 but may increase the bleeding time to a greater extent if other, mild hemostatic deficiencies (e.g., vWD) are present. Aspirin resistance caused by genetic or acquired mechanisms occurs in approximately 10% of patients with acute coronary syndromes.
37
Major surgery, including cardiac surgery, in patients with aspirin-inhibited platelets may increase perioperative bleeding.
36,
38,
39,
40 Nearly all patients presenting with acute coronary syndromes receive aspirin as part of their initial therapy, but usually the impact on surgical hemostasis is minor.
38 Aspirin-related bleeding is treated by platelet transfusions, since aspirin is cleared from blood in <90 minutes.
41Clopidogrel and more recently prasugrel are thienopyridines that are irreversible platelet inhibitors that prevent adenosine diphosphate binding to platelet P2Y
12 receptors. Both drugs have rapid platelet inhibition, but the kinetics of prasugrel are faster and more consistent and the drug is preferred for management of acute coronary syndromes.
42,
43 Median plasma half-life is 7 to 8 hours, but platelet inhibitory effects vary and last 3 to 10 days after the drug is discontinued.
33 Both drugs are routinely used in combination with aspirin to reduce adverse cardiac ischemic events.
44 Major bleeding complications from the combination of aspirin and prasugrel are slightly, but significantly higher (2.4%) than with aspirin and clopidogrel (1.8%).
42 For this reason, the Society of Thoracic Surgeons’ guidelines recommend stopping thienopyridines at least 3 days and preferably 5 days before elective coronary arterial bypass surgery.
39 However, reversal of thienopyridines is highly variable between patients. A point-of-care device, which assesses platelet responsiveness to ATP, showed reduced platelet aggregation in some patients who had stopped thienopyridines within 7 days of operation.
45Platelet GPIIIa/IIb (α
IIbβ
3) receptor antagonists (abciximab, eptifibatide, and tirofiban) inhibit most platelet functions and prolong bleeding times considerably longer than aspirin.
46 Abciximab (Reopro) rapidly binds to the platelet receptors and remains bound for the life of the platelet.
47 Eptifibatide and tirofiban are reversible inhibitors and relatively short acting. Inhibitory effects of abciximab last approximately 48 hours, whereas that of eptifibatide lasts <1 hour.
47 Certain prostaglandins inhibit platelets by activating platelet adenylate cyclase or inhibiting cyclic nucleotide phosphodiesterases (e.g., prostacyclin and dipyridamole).
33 All of these platelet inhibitors increase the bleeding time.
33Warfarin. For minor or superficial operations, it is usually not necessary to reverse warfarin, but for major operations, warfarin should be stopped for 3 to 5 days before surgery, if the likelihood of a clotting event within that interval is low.
13 The PT should be checked before operation to be sure that most, if not all, of the warfarin effect has been reversed. In patients that cannot safely tolerate interruption of anticoagulation (e.g., mechanical heart valves), intravenous heparin may be used to maintain anticoagulation until 2 to 3 hours before incision.
Oral vitamin K (2 to 4 mg) administration usually corrects the PT to normal within 24 hours.
48 In an emergency, warfarin anticoagulation can be reversed by intravenous vitamin K (10 mg IV, infused slowly over 30 minutes) and plasma infusion (15 to 30 mL/kg, or 4 to 8 units in an adult) over 4 to 6 hours, with careful attention to volume status of the patient.
49,
50) Transfusions of prothrombin complex concentrates (PCCs), some of which contain high concentrations of vitamin K-dependent coagulation factors II, VII, IX, and X, have also been utilized. PCC acts rapidly but may have a risk of thrombosis.
51,
52 If replacement therapy is not effective, administration of recombinant factor VIIa (rFVIIa) (2 mg IV, or 20 to 40 µg/kg for 100 to 50 kg patients) is another alternative in patients with emergency or life-threatening bleeding
39,
53; however, this agent also has defined risks of arterial thrombotic complications, particularly in the elderly or at higher doses.
54Dabigatran is a recently approved, oral inhibitor of thrombin that does not require blood monitoring.
55,
56,
57 The drug is taken twice daily and is effective prophylaxis in patients with paroxysmal or chronic atrial fibrillation (AF),
58 in treatment of acute venous thromboembolism,
57 and in acute coronary
syndromes.
59 Plasma half-life is 12 to 17 hours,
56 and the drug is cleared by the kidney.
Rivaroxaban is a new, oral factor Xa inhibitor that is rapidly absorbed and has a half-life of 9 to 12 hours and can be given as a fixed dose.
59,
60. Most of the drug is eliminated in urine and the remainder in feces. Rivaroxaban has been approved for preventing stroke in patients with AF (ROCKET-AF trial),
61 as it reduced the incidence of stroke and intracranial bleeding compared with warfarin treatment (0.49 vs. 0.74%). If emergency surgery is required before either dabigatran or rivaroxaban is eliminated, rFVIIa may be attempted to control bleeding, but its value is not certain as an antidote for anticoagulants.
62 Fibrinolytic drugs. If operation occurs within 12 hours of the last dose of streptokinase or tissue-plasminogen activator (t-PA), the risk of surgical bleeding is increased.
63 If excessive bleeding occurs in such patients, plasma fibrinogen can be replaced with cryoprecipitate, fresh frozen plasma, or fibrinogen concentrate (available in Europe). Platelet transfusion may also be needed, especially if a long-acting platelet inhibitor such as a thienopyridine or abciximab is circulating. Antifibrinolytic drugs are not generally utilized, as the thrombolytic agent will have been cleared from the circulation in most cases. Fibrinolytic drugs are cautiously recommended for treating thrombotic strokes. Potential benefits appear to outweigh the risk of intracranial hemorrhage or surgical site bleeding.
64,
65,
66