Fig. 16.1
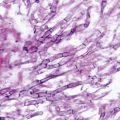
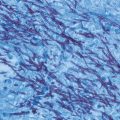
The mycelial phase of B. dermatitidis (left) produces no unique characteristics which allow organism identification. As a yeast (right), B. dermatitidis has characteristic thick walled, multinucleated cells with single broad-based daughter cells
Primary isolation of B. dermatitidis from clinical specimens is most reliable when grown as the mycelial form at 30 °C. Mycelial colonies, which are white to brown in color, grow on agar in 1–3 weeks. Positive identification of B. dermatitidis requires conversion to the yeast form at 37 °C or nucleic acid amplification methods which allow early identification of mycelial phase growth. Yeast-like colonies are wrinkled and cream-to-tan in color. Asexual reproduction is by budding of single, broad-based, thick walled, multinucleated daughter cells (Fig. 16.1). The same morphologic characteristics are observed in tissue samples from infected individuals. In the appropriate clinical situation, this allows a presumptive diagnosis of blastomycosis [6].
Genotypic Characteristics
A. dermatitidis, the telomorphic form of B. dermatitidis, has been shown to have heterothallic mating-type cultures [5, 6]. Mating is controlled by a highly conserved domain that determines plus (MAT1) and minus (MAT2) strains. The complete sexual cycle has been defined by the DNA structure of the MAT locus (alpha or high mobility group, HMG, domain), mating assays, and genetic recombination [7]. The B. dermatitidis MAT locus is similar to those of other dimorphic fungi in which the mating-type gene is linked to APN2, SLA2, and COX13 genes [7].
A variety of nucleic acid techniques have been employed to examine the genetic diversity and geographic distribution of B. dermatitidis isolates. Polymerase chain reaction (PCR)-based assays targeting the promoter region of the BAD-1 virulence gene identified two major genotypic groups A and B, representing 47.2 and 48.1 %, respectively, and three minor groups (C, D, and E) in an analysis of 106 clinical and environmental isolates of B. dermatitidis from Wisconsin, Georgia, and Africa [8]. Recently, the technique of microsatellite analysis has been applied to a number of fungal species as well as B. dermatitidis [9–13]. Microsatellite analysis offers several advantages over both PCR-restriction fragment length polymorphism (RFLP) and random amplified polymorphic DNA [14, 15] (RAPD) methods. A panel of 27 microsatellite markers distinguished 112 geographically diverse isolates into two genetically distinct B. dermatitidis groups [13]. Group 1 isolates were virtually monomorphic (1.8 alleles/locus) compared to Group 2 which were highly diverse (8.2 alleles/locus). The α and HMG mating types were almost evenly divided between the two groups [13].
The identification of species by sequencing a genetic marker has become common practice among mycologists [14, 16]. Genealogical concordance phylogenetic species recognition (GCPSR) techniques have been used to identify populations, delineate cryptic species, and document genetic recombination and gene flow in a number of fungal pathogens [9, 11, 17–20]. GCPSR employing seven nuclear genes to evaluate 78 human, canine, and environmental B. dermatitidis isolates from diverse geographic regions also revealed two distinct monophyletic clades within B. dermatitidis, phylogenetic species 1 (PS1, clade 1) and phylogenetic species 2 (PS2, clade 2), which may represent a genetically divergent novel cryptic species [21]. Although only a few isolates were subjected to both multilocus satellite analysis and GCPSR, GCPSR-defined PS1 and PS2 correspond to the highly diverse Group 2 and monomorphic Group 1 as described by Meece et al. [13].
Epidemiology
The ecological niche of B. dermatitidis has not been conclusively established. Environmental isolations of the organism associated with disease outbreaks indicate that the organism grows as microfoci in warm, moist soil in wooded areas that are rich in organic material [22–24]. Analysis of sporadic cases in humans and dogs, point source outbreaks, and infrequent environmental isolations has provided the major basis for the definition of endemic regions in North America [3, 4]. B. dermatitidis is endemic to the eastern USA, the Mississippi, and Ohio River valleys, extending northward to the Great Lakes and southern Canada. While most cases have been reported in Mississippi, Arkansas, Kentucky, Tennessee, and Wisconsin, up to 14 % of blastomycosis cases identified from Medicare claims data occurred outside the endemic region [25, 26]. Within these known endemic regions there exist hyperendemic areas with exceptionally high attack rates [3, 4].
Other mammals, especially dogs, may become infected [27]. Early studies of endemic cases suggested middle-aged men with outdoor occupations were at greatest risk for blastomycosis. Subsequent reviews of reported outbreaks indicate no predilection for sex, age, race, occupation, or seasonal exposure [3, 4]. Vocational or avocational exposure to soil appears to be a common factor associated with both endemic and epidemic disease.
Pathogenesis and Immunology
Blastomycosis is initiated by the inhalation of the conidia of B. dermatitidis. Following inhalation, the infectious conidia are nonspecifically phagocytosed and killed by polymorphonuclear leukocytes (PMN), monocytes, and alveolar macrophages. This phagocytic response represents natural or innate immunity and may in part explain asymptomatic cases observed in outbreaks. Conidia which escape the initial phagocytic response rapidly convert to a yeast form that is more resistant to phagocytosis and killing. In in vitro studies, B. dermatitidis yeast evades macrophage defenses [28] and suppresses nitric oxide production by inhibition of inducible nitric oxide synthase [29]. Several virulence factors have been associated with the pathogenicity of B. dermatitidis. The thick cell wall of the yeast has been proposed to have antiphagocytic properties. Higher concentrations of lipids and phospholipids in cell walls in some strains have been associated with increased virulence.
Conversion of B. dermatitidis to the yeast form induces the expression of a yeast phase-specific gene designated BAD-1 (formerly WI-1). BAD-1 (WI-1) is a 120-kDa glycoprotein adhesion and immune modulator with a number of essential properties, including CR3 and CD14+ binding, and an epidermal growth factor (EGF)-like domain [30–33]. The protein contains a repetitive domain that mimics thrombospondin type-1 (TSP-1) and suppresses T lymphocyte activation and effector function by binding heparin sulfate [34]. BAD-1 is a major virulence factor and its deletion results in attenuated pathogenicity [35, 36].
Cellular immunity in humans, as determined by antigen-induced lymphocyte proliferation, has been documented using whole yeast phase organisms, an alkali-soluble, water-soluble yeast extract, and BAD-1 [3, 4]. As with other endemic fungi, B. dermatitidis seems to require type 1-dependent cell-mediated immunity (CMI) [37]. Recent vaccine studies in animal models have shown that CMI is mediated by both vaccine-induced CD4+ [37] and CD8+ T cells [38] which produce type-1 cytokines such as gamma interferon (IFN-γ) and tumor necrosis factor alpha (TNF-α). In addition, the CD28+ T cell receptor has been shown to be required for the induction of protective T cell responses to B. dermatitidis infection [39]. CD4+ cells require interleukin-12 (IL-12) for the development of CMI while CD8+ cells were less dependent on IL-12 for this process [40].
Development of a vaccine to prevent blastomycosis is a major goal but is hampered by an incomplete understanding of the immune response. Bronchoalveolar macrophage (BAM) activity is limited for fungal pathogens such as B. dermatitidis [41]. Binding of the yeast cells to surfactant protein D results in interference with BAM TNF-α production, blunting the host defense [42]. This may be compensated by the stimulation of BAM TNF-α by the abundant 1,3-β-glycan on the B. dermatitidis yeast cell. Intradermal administration of an attenuated B. dermatitidis lacking BAD-1 protects mice against lethal pulmonary challenge but intranasal vaccine delivery fails to do so [37]. Mucosal vaccination leads to poor T cell activation by induction of matrix metalloproteinase two, which impairs the chemokine response [35]. These studies in mice [37] and dogs [35] are promising and indicate that the development of a vaccine to prevent disease in humans is possible.
Clinical Manifestations
The clinical manifestations of blastomycosis are varied and include asymptomatic infection, acute or chronic pneumonia, and extrapulmonary disease. A number of factors are known to influence clinical presentation of disease. Patient characteristics and comorbidities as well as genetic variations of the infecting organism affect disease outcome. Univariate analysis of 16 clinical disease and patient demographic characteristics showed significant associations with different groups of B. dermatitidis [13, 43]. The monomorphic group 1 B. dermatitidis isolates identified by microsatellite analysis were more likely to be associated with pulmonary-only infections, while the more genetically diverse group 2 isolates were more likely to disseminate [43]. Group 2 isolates were more frequently seen in older patients who were smokers and had a comorbid condition [43].
Extrapulmonary disease results from the hematogenous spread of the fungus from a primary pulmonary infection. Although extrapulmonary B. dermatitidis infection has been reported to involve almost every organ of the human body, the skin, bones, and genital urinary system are most common (Table 16.1). It is important to note that blastomycosis mimics many other disease processes, whether acute or chronic [3]. For example, acute pulmonary blastomycosis is often mistaken for bacterial community-acquired pneumonia or influenza. Chronic pulmonary blastomycosis commonly mimics a malignancy or tuberculosis. Skin lesions are often misdiagnosed as pyoderma gangrenosa, or keratoacanthoma. Blastomycosis of the larynx is frequently misdiagnosed as carcinoma. Thus, a high index of suspicion and a careful histologic evaluation of secretions or biopsy material is needed.
Table 16.1
Organ involvement in blastomycosis
Organ system involved | No. involved/total patients (%) |
|---|---|
Pulmonary | 369/534 (69) |
Cutaneous | 306/534 (57) |
Osseous | 116/534 (22) |
Genitourinary | 92/534 (17) |
Central nervous system | 29/534 (5) |
Pulmonary Blastomycosis
Acute Infection
Initial infection occurs after inhalation of conidia into the lungs. Unless associated with an outbreak or group exposure, acute infection is frequently unrecognized. Clinical studies involving point-source outbreaks of infection indicate that symptomatic acute pulmonary disease occurs in only 50 % of individuals, usually after an incubation period of 30–45 days [22, 23]. Signs and symptoms of acute pulmonary blastomycosis are similar to those of influenza or bacterial pneumonia. Fever, chills, pleuritic chest pain, arthralgias and myalgias usually occur abruptly. At onset, cough is nonproductive but frequently becomes purulent as disease progresses. Chest radiographs commonly reveal alveolar infiltrates with consolidation (Fig. 16.2) [3, 4]. Pleural effusions are uncommon and, if present, are typically small in volume. Hilar adenopathy is uncommon and is a useful sign in distinguishing acute blastomycosis from acute histoplasmosis. Spontaneous cures of symptomatic acute infection have been documented, but the exact frequency of these cures has not been clearly established [3, 4]. Although not an opportunistic infection, immunosuppression is a risk factor for serious pulmonary complications such as acute respiratory distress syndrome (ARDS) [3, 4].
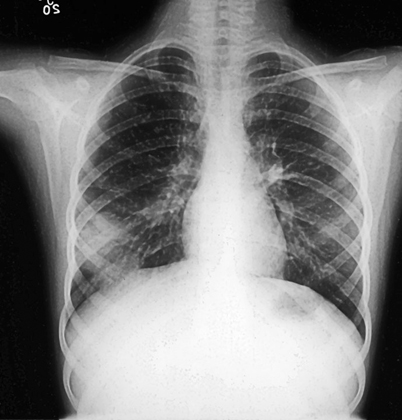

Fig. 16.2
Acute pulmonary blastomycosis. Chest radiograph reveals peripheral alveolar infiltrate which appear to be pleural based. Although this patient clinically improved with antibiotics sputum culture grew B. dermatitidis
Chronic Infection
The majority of patients diagnosed with pulmonary blastomycosis have a chronic pneumonia, which is clinically similar to tuberculosis, other fungal infections, and cancer. Symptoms include fever, weight loss, chronic productive cough, and hemoptysis. The most frequent radiologic findings are alveolar infiltrates (Fig. 16.3) with or without cavitation, mass lesions that mimic bronchogenic carcinoma (Fig. 16.4), and fibronodular infiltrates [3, 4]. Although small pleural effusions have been reported, large pleural effusions (Fig. 16.5) are distinctly uncommon and, when present, have been associated with poor outcome.
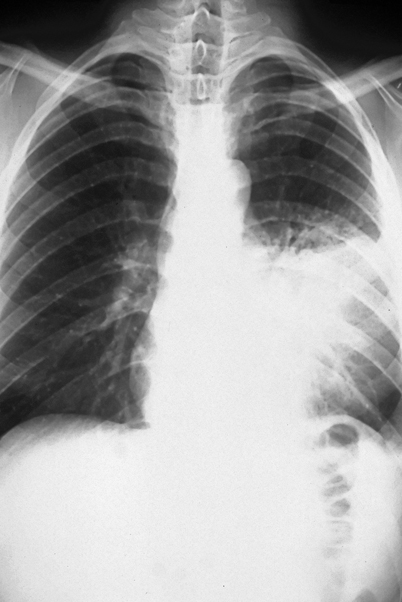
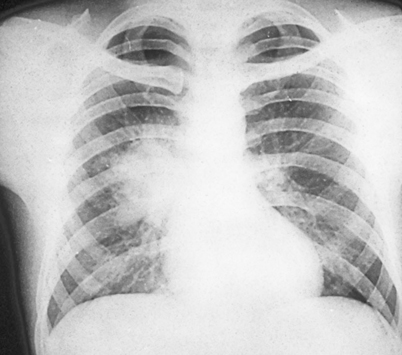
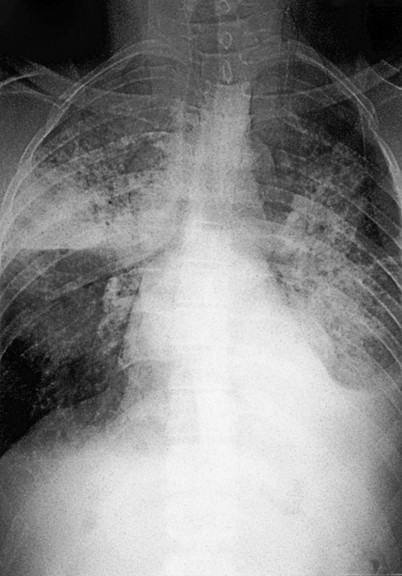

Fig. 16.3
Progressive pulmonary disease showing extensive left mid-lung alveolar infiltrate. This patient failed multiple courses of oral and intravenous antibiotics over a 2 month period prior to diagnosis of blastomycosis

Fig. 16.4
Chest radiograph with right hilar infiltrate that mimics bronchogenic carcinoma. Bronchoscopy with biopsy and pulmonary cytology should be performed in these patients presenting with this radiographic finding to rule out concomitant disease

Fig. 16.5
Patient with life threatening pulmonary disease whose chest radiograph reveals bilateral alveolar infiltrates and large left sided pleural effusion
Acute Respiratory Distress Syndrome
Patients may occasionally present with ARDS associated with miliary disease or diffuse pneumonitis (Fig. 16.6). Mortality exceeds 50 % in these patients and most deaths occur within the first few days of therapy [44]. Diffuse pulmonary infiltrates and respiratory failure are more likely to occur in immunocompromised patients, especially those with late-stage AIDS [3, 4].
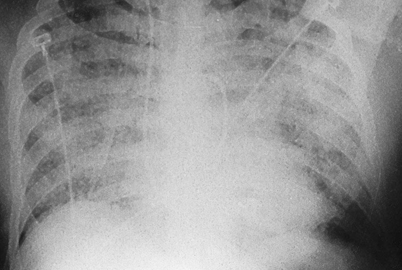

Fig. 16.6
Diffuse pulmonary infiltrates in a patient with ARDS. Patients presenting with this syndrome have a mortality rate greater than 50 %
Extrapulmonary Blastomycosis
Extrapulmonary disease has been reported in as many as two thirds of patients with chronic blastomycosis. This high frequency probably reflects selection bias as these figures were reported in earlier autopsy-based studies before effective therapy was available [3, 4]. More recent studies have documented extrapulmonary disease in only 25–40 % of patients with blastomycosis [44]. Extrapulmonary disease is almost always seen in conjunction with active pulmonary disease.
Skin Disease
Skin disease is the most common extrapulmonary manifestation of blastomycosis. Two types of skin lesions occur, verrucous and ulcerative (Fig. 16.7). The verrucous lesion is most common, typically with well-demarcated borders from gray to violaceous in color. These lesions may mimic squamous cell carcinoma. Microabscesses develop at the periphery of these lesions. Specimens taken from the margins usually reveal the diagnostic yeast form on wet preparation (Fig. 16.8). The second type of lesion is ulcerative. These ulcers are friable and bleed easily and usually have well-demarcated, heaped-up borders. The ulcers of blastomycosis develop from subcutaneous pustular lesions which spontaneously rupture and drain.
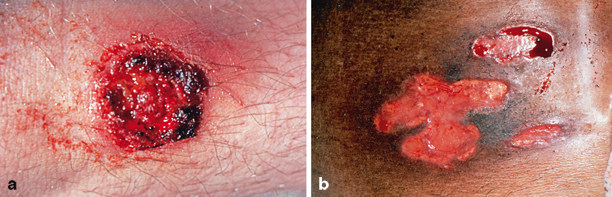
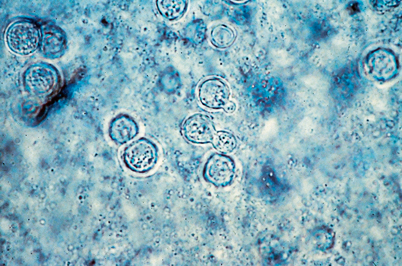

Fig. 16.7
Cutaneous blastomycosis typically produces verrucous (left) or ulcerative (right) lesions

Fig. 16.8
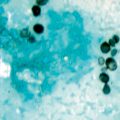
Diagnosis of blastomycosis. This figure shows the characteristic yeast forms in a wet preparation of a skin scraping. Scraping of the edges of the verrucous and ulcerative lesions yield the best diagnostic results
Regional lymphadenopathy is usually not present in cases of pulmonary dissemination. Inoculation blastomycosis following a dog bite or autopsy accidents often have lymphadenopathy/adenitis as a prominent feature [3, 4]. Lesions may also appear on the mucosa of the nose, mouth, and larynx. Laryngeal blastomycosis mimics well-differentiated squamous cell carcinoma both clinically and histopathologically. Subcutaneous nodules or cold abscesses may be seen in patients with multiorgan involvement.
Osseous
Osteomyelitis occurs with as many as one fourth of B. dermatitidis infections [3, 4]. Although any bone may be affected, the vertebrae, pelvis, sacrum, skull, ribs, or long bones are most frequently involved. Granuloma formation, suppuration, or necrosis is observed in biopsy specimens. A well-circumscribed osteolytic lesion may be observed on radiographs. Such lesions are radiographically indistinguishable from other fungal, bacterial, or neoplastic disease. Patients with B. dermatitidis osteomyelitis usually present with contiguous soft tissue abscesses or chronic draining sinuses. Although most bone lesions resolve with prolonged antifungal therapy, some may require surgical debridement for cure.
Genitourinary
In men, 10–30 % of blastomycosis involves the genitourinary tract, primarily the prostate and epididymis [3, 4]. Prostatic involvement is frequently associated with symptoms of obstruction, and includes an enlarged, tender prostate and pyuria. Urine cultures obtained following prostate massage are frequently positive. Female genitourinary blastomycosis is rare but may include endometrial infection and tubo-ovarian abscess.
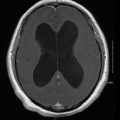
Central Nervous System
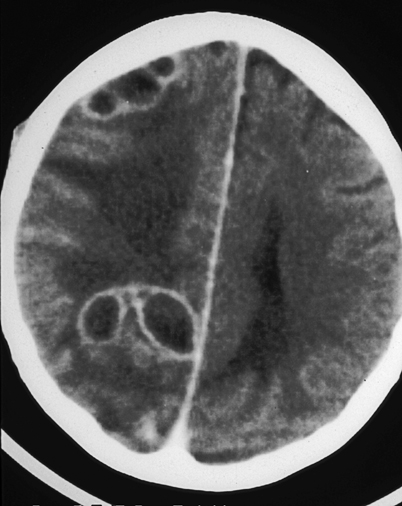
CNS involvement occurs in less than 5 % of cases in immunocompetent patients. Persons with AIDS have rates of CNS involvement as high as 40 % [45] and other studies confirm immunosuppression as a risk factor for dissemination [46]. CNS blastomycosis may present as an abscess (epidural, cranial, or spinal) or as meningitis (Fig. 16.9) [46]. Magnetic resonance imaging (MRI), alone or in conjunction with computed tomography scans, reveals CNS abnormalities and are used to identify CNS involvement [46, 47]. Surgical intervention may be necessary both for diagnosis and to prevent neurologic deterioration [48].