| Microbe/toxin | Disease |
|---|---|
| Bacillus anthracis | Anthrax – Inhalational, cutaneous, injection |
| Variola virus | Smallpox and its variants |
| Yersinia pestis | Plague – Pneumonic, bubonic, septicemic |
| Clostridium botulinum | Botulism |
| Francisella tularensis | Tularemia – Pneumonic, typhoidal |
| Viral hemorrhagic fevers | |
| Filoviruses | Ebola, Marburg |
| Arenaviruses | Lassa fever, South American hemorrhagic fevers |
| Bunyaviruses | Rift Valley fever, Congo–Crimean hemorrhagic fever |
Based upon potential morbidity, aerosol feasibility, dissemination characteristics, and diagnostic difficulty
| Microbe/toxin | Disease |
|---|---|
| Coxiella burnetii | Q fever |
| Brucella species | Brucellosis |
| Burkholderia mallei | Glanders |
| Burkholderia pseudomallei | Melioidosis |
| Chlamydia psittaci | Psittacosis |
| Rickettsia prowazekii | Epidemic typhus |
| Alphaviruses | Viral encephalitides |
| Ricin | Ricin intoxication |
| Staphylococcus enterotoxin B | Staphylococcal toxin illness |
| Salmonella species, Shigella dysenteriae, Escherichia coli 0157;H7, Vibrio cholerae, Cryptosporidium parvum | Food- and waterborne gastroenteritis |
Based upon potential for production and dissemination, availability, morbidity/mortality
| Microbe/toxin | Disease |
|---|---|
| Hantaviruses | Viral hemorrhagic fevers |
| Flaviviruses | Yellow fever |
| Mycobacterium tuberculosis | Multidrug-resistant tuberculosis |
| Nipah virus | Systemic, flu-like illness |
Other examples of candidate threat agents that possess some elements of bioterrorism concern
| Genetically engineered vaccine- and/or antimicrobial-resistant Category A or B agents |
| Human immunodeficiency virus 1 |
| Adenoviruses |
| Influenza |
| Rotaviruses |
| Hybrid pathogens – e. g., smallpox/plague, smallpox/Ebola |
Clinical presentation
The clinical pictures of diseases caused by agents of BT are varied but, with few exceptions among the CDC category A and B threats, can be categorized into a limited number of syndromic presentations (Table 122.2). Unfortunately, there are scant pathognomonic features of BT-related illness, only suggestive ones. For this reason a high index of suspicion among clinicians is necessary to capitalize on subtle clues, many of which may be epidemiologic in nature. Nonetheless, many of these agents demonstrate suggestive clinical and laboratory findings (Table 122.2) that in the appropriate clinical context should prompt further, targeted evaluations and warrant appropriate, empiric management. More detailed clinical information on each agent is covered in organism-specific chapters of this book.
| Syndrome | Clinical presentation | Differential diagnosis | BT-associated disease | Disease-specific clues |
|---|---|---|---|---|
| Influenza-like illness | Nonspecific constitutional and upper respiratory symptoms: malaise, myalgias, nausea, emesis, dyspnea, cough +/− chest discomfort, without coryza or rhinorrhea → abrupt onset of respiratory distress +/− shock +/− mental status changes, with CXR abnormalities (wide mediastinum or infiltrates or pleural effusions) | Influenza, community-acquired bacterial pneumonia, viral pneumonia, Legionella, Q fever, psittacosis, mycoplasma, Pneumocystis pneumonia, tularemia, dissecting aortic aneurysm, bacterial mediastinitis, SVC syndrome, histoplasmosis, coccidioidomycosis, sarcoidosis, ricin, and Staphylococcus enterotoxin B (pulmonary edema/ARDS) | Inhalational anthrax |
|
| Skin lesions | Pruritic, painless papule on exposed areas→vesicle(s)→ ulcer→edematous black eschar +/− massive local edema and regional adenopathy, +/− fever, evolving over 3–7 days | Recluse spider bite, staphylococcal lesion, atypical Lyme disease, Orf, glanders, tularemia, plague, rat-bite fever, ecthyma gangrenosum, rickettsialpox, atypical mycobacteria, cutaneous diphtheria, cutaneous leishmaniasis | Cutaneous anthrax |
|
| Fulminant pneumonia | Abrupt-onset constitutional symptoms and rapidly progressive respiratory illness with cough, fever, rigors, headache, sore throat, myalgias, dyspnea, pleuritic chest pain, GI symptoms, lung consolidation, +/− hemoptysis, +/− shock; variable progression to respiratory failure | Severe community-acquired bacterial or viral pneumonia, inhalational anthrax, pulmonary infarct, pulmonary hemorrhage, influenza, mycoplasma pneumonia, Legionella, Q fever, SARS, tuberculosis |
|
|
| Sepsis with bleeding diathesis and capillary leak | Sepsis syndrome, GI symptoms, mucosal hemorrhage, altered vascular permeability, DIC, purpura, acral gangrene, hepatitis, hypotension, +/− CNS findings, multiorgan system failure | Meningococcemia; gram-negative sepsis, streptococcal, pneumococcal, or staphylococcal bacteremia with shock; malaria, leptospirosis, typhoid fever, borrelioses, typhoidal tularemia; overwhelming postsplenectomy sepsis; acute leukemia; Rocky Mountain spotted fever; fulminant hepatitis, TTP, hemolytic-uremic syndrome, SLE, hemorrhagic smallpox; hemorrhagic varicella (in immunocompromised) |
|
|
| Febrile prodrome with generalized exanthem | Fever, malaise, prostration, headache, myalgias, and enanthema followed by development of synchronous, progressive, centrifugal papular→vesicular→pustular rash on face, mucous membranes, extremities>>trunk→generalization +/− hemorrhagic component, with systemic toxicity | Varicella, drug eruption, Stevens–Johnson syndrome, measles, secondary syphilis, erythema multiforme, severe acne, disseminated herpes zoster or simplex, meningococcemia, monkeypox, generalized vaccinia related to smallpox vaccination, insect bites, Coxsackievirus, vaccine reaction | Smallpox |
|
| Progressive weakness | Acute onset of afebrile, symmetric, descending flaccid paralysis that begins in bulbar muscles, dilated pupils, diplopia or blurred vision, dysphagia, dysarthria, ptosis, dry mucous membranes→airway obstruction + respiratory muscle paralysis, clear sensorium and absence of sensory changes | Myasthenia gravis, brainstem CVA, polio, Guillain–Barré syndrome variant, tick paralysis, chemical intoxication | Botulism |
|
Abbreviations: CXR = chest x-ray; SVC = superior vena cava; ARDS = acute respiratory disease syndrome; CT = computed tomography; GI = gastrointestinal; SARS = severe acute respiratory syndrome; DIC = disseminated intravascular coagulation; CNS = central nervous system; TTP = thrombotic thrombocytopenic purpura; SLE = systemic lupus erythematosus; VHF = viral hemorrhagic fever; CVA = cerebrovascular accident.
Diagnosis
Rapid detection and accurate identification of BT agents are important not only for confirming that a BT event has occurred but also for treating individual patients and implementing appropriate public health measures. By definition BT is insidious; in the absence of credible advance notification, it is likely that clustered, syndromic, clinical illness will be the initial manifestation of a BT attack. Early recognition, although critical, is problematic for a variety of reasons: (1) targets of BT, especially in an open society, are diverse and unpredictable; (2) the clinical latency of BT agents, discussed above, makes it likely that clusters of symptomatic individuals will present for medical care days to weeks after an “event” and at geographically diverse locations; (3) initial clinical manifestations of many BT-related illnesses are nondiagnostic and may be mistaken for other, more common but less impactful diagnoses; (4) clinicians are inexperienced with the clinical manifestations of these infections; and (5) even if the classic clinical findings are known, because BT agents are manipulated in the laboratory, their associated clinical syndromes may not present in the same manner as those from naturally occurring infection. Conversely, in the setting of a high level of suspicion, early recognition may be aided by a number of epidemiologic and clinical clues: case clustering, which because of the clinical latency of BT requires attention to geographic surveillance and communications; unusual clinical presentations of common syndromes, such as fulminant pneumonia in otherwise healthy young adults; or unusual disease patterns, such as rare diseases occurring in nonendemic areas or concurrent disease in humans and animal populations.
The CDC has developed a national laboratory response network (LRN) for BT that integrates selected microbiologic laboratories across the United States into a network and mandates uniform practices for specimen collection, processing, shipping, security, and testing. Laboratories within the LRN consortium are designated as having screening, confirmatory, or reference functions. The network of laboratories is connected by a secure communications system, thus ensuring the timely flow of information among the CDC, other governmental agencies, state health authorities, and other laboratories. The mission of the LRN is to enable a rapid and organized response to BT; the CDC routinely audits the performance of network members using panels of unknown pathogens.
Although diagnostic tests are available for most BT agents, many are not readily available in clinical laboratories, are time-consuming, have less than optimal sensitivity and specificity, or cannot test for multiple agents simultaneously. Diagnostic platforms that can assess for the presence of multiple pathogens concurrently, so-called multiplex strategies, offer attractive advantages, especially in the arenas of environmental surveillance and in screening either patients presenting with nonspecific symptoms or asymptomatic individuals who have possible exposure to an unknown agent.
The preferred methods for the laboratory diagnosis of BT agents differ depending on the agent in question. For most bacterial agents the gold-standard diagnostic assay remains standard culture; other supporting assays include modified staining with light microscopy, motility testing, lysis by gamma phage, capsule production staining, hemolysis, wet mounts, staining for spores, slide agglutination, direct fluorescent antibody, enzyme-linked immunosorbent assay (ELISA), and rapid immunochromatography. Routine assays for viral agents include virus isolation through tissue culture or growth in eggs, direct and indirect immunofluorescence, immunodiffusion in agar, electron microscopy, modified staining and light microscopy, plaque reduction neutralization, hemagglutination inhibition, neuraminidase activity, complement fixation, and ELISA. Pathologic examination of tissues and immunohistochemistry also play an important role in diagnosing BT agents.
Molecular assays are becoming the new gold standard for BT detection, with sensitivities and specificities close to 100% when compared with culture or serologic assays. These assays detect infectious agents in humans through target nucleic acid isolation and amplification followed by specific pathogen identification. Several technologies and methods have been used for multiplex detection of different BT agents. Although still in the developmental stages, it is likely that DNA microfluidic devices will be commonly used diagnostic platforms in the future. These methods are sensitive and specific and theoretically can be used on unprocessed specimens in field settings, thus obviating laborious microbial isolation steps. However, several challenges, including sampling issues, data analysis, development of specific probes, quality control, cost containment, automation, performance, and integration, must be addressed before such methods replace the standard ones.
Laboratory diagnosis of specific Category A agents
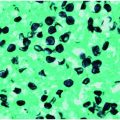
Bacillus anthracis (Figures 122.1 and 122.2)
Presumptive laboratory identification of Bacillus anthracis is based on the presence of large gram-positive bacilli in either gram- or
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree