Definitions
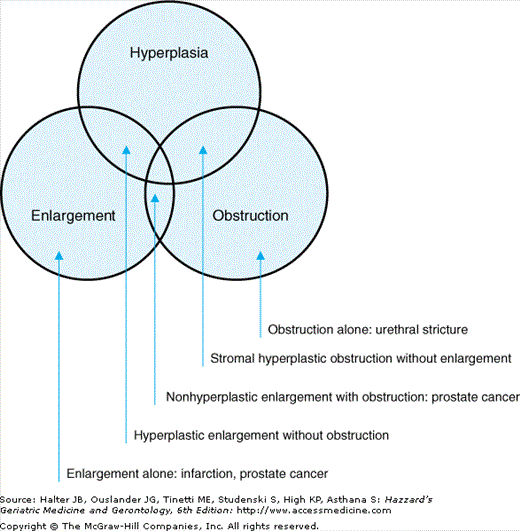
Many terms are used to describe benign prostate disease, and often interchangeably. Precision is important, however, because the conditions overlap only partially (Figure 50-1). Benign prostatic hyperplasia (BPH) is a histological condition characterized by benign proliferation of stromal and/or epithelial prostate tissue. Benign prostate enlargement (BPE) occurs in about half of men with BPH, and is quantified by milliliters of prostate tissue. Bladder outlet obstruction (BOO) occurs in only a subset of men with BPE. Older men often have voiding symptoms (urgency, frequency, nocturia, slow stream, hesitancy, incomplete emptying, postvoiding dribbling, and incontinence), which maybe related to BPH, BPE, BOO, age-related physiologic changes in the lower urinary tract, or comorbid conditions and medications. Therefore, voiding symptoms are best described by the nonspecific term lower urinary tract symptoms (LUTS).
Epidemiology and Natural History
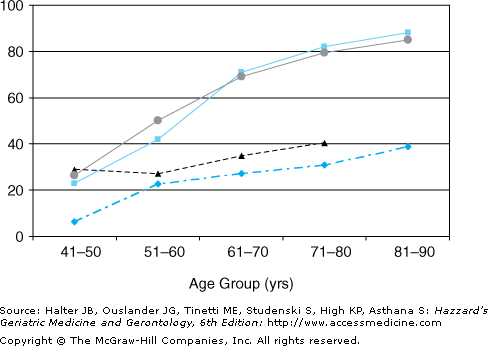
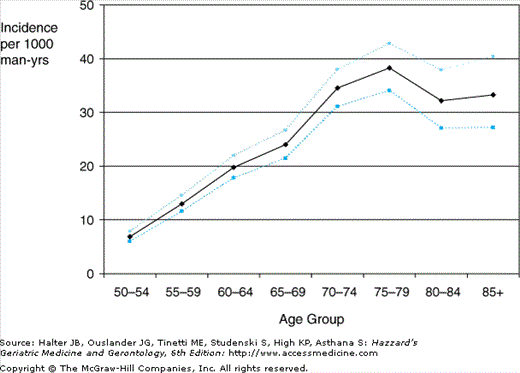
Early autopsy and epidemiological studies demonstrated a marked rise in prevalence of BPH and BPE with age, especially during the sixth and seventh decades, and a similar relationship between age and “clinical BPH” (LUTS and BPE on examination) (Figure 50-2). Overall, 28% to 35% of older men without previous prostate surgery have moderate to severe LUTS. More recent data from longitudinal epidemiological studies and placebo arms of treatment trials confirm that the incidence of benign prostate disease gradually and variably increases with age until the ninth decade (Figure 50-3). Only weak correlations exist between BPE, BOO, and LUTS, suggesting that their relationships are nonlinear and complex.
Figure 50-2.
Autopsy evidence of prostate hyperplasia (percent of prostates, solid line with ▪), mean weight (mL) of all prostates (dashed line with ♦), mean weight of prostates with hyperplasia (dashed line with ▴), and percent of men with lower urinary tract symptoms (solid line with ○). Data from Berry SJ, Coffey DS, Walsh PC, Ewing LL. The development of human benign prostatic hyperplasia with age. J Urol. 132:474–479, 1984; Guess HA, Arrighi HM, Metter EJ, Fozard JK. Cumulative prevalence of prostatism matches the autopsy prevalence of benign prostatic hyperplasia. Prostate. 17:214–216, 1990.
Figure 50-3.
Incidence per 1000 man-years of BPH, as defined by LUTS plus a clinical diagnosis of BPH, medical therapy for BPH, or surgical treatment for BPH during follow-up. Data from: Verhamme KMC, Dieleman JP, Bleumink GS, et al. Triumph Pan European Expert Panel. Incidence and prevalence of lower urinary tract symptoms suggestive of benign prostatic hyperplasia in primary care—the Triumph project. Eur Urol. 42:323–328, 2002.
Some data suggest that African-American men have a higher risk of benign prostate disease urinary retention, and BPE/BOO surgery, possibly because they have higher levels of 5α-reductase and larger prostate transitional zone volume (see section “Pathophysiology”). The very limited available data suggest that Hispanic men have similar risk of BPH and characteristics of BPE as non-Hispanic white men. Asian men generally have lower rates of benign and malignant prostate disease, although this may differ by country of origin.
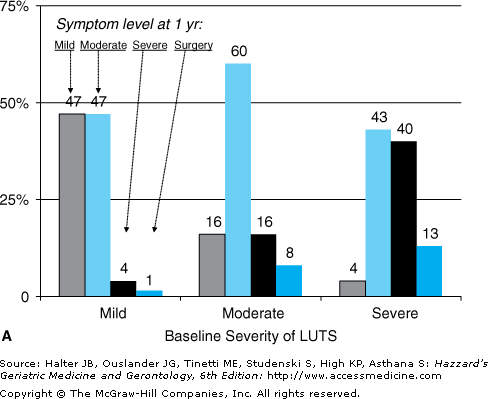
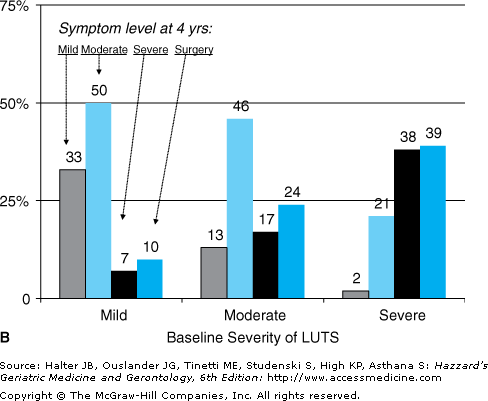
Progression of benign prostate disease is defined as worsening LUTS, acute urinary retention (AUR), and/or the need for surgical treatment. LUTS can vary significantly over time, and abate as well as progress. In a prospective cohort of men with moderate LUTS, by 1-year symptoms had improved in 16% and worsened in only 24%, and at 4 years, symptoms were only mild in 13%, unchanged in 46%, and worse in 41% (Figure 50-4). The incidence of AUR in men with LUTS and benign prostate disease is low: in a meta-analysis including 6100 moderately symptomatic men, the incidence of AUR was 13.7 per 1000 patient-years in all men and considerably higher in men aged ≥70 years or taking anticholinergic agents (34.7 per 1000 patient-years). Higher baseline prostate-specific antigen (PSA) levels are associated with greater risk of AUR and surgery; 7.8% of men with PSA levels up to 1.3 ng/mL develop AUR and/or require surgery by 4 years, compared with 12.6% of men with PSA levels 1.4 to 3.2 ng/mL, and 19.9% with PSA levels 3.3 to 12 ng/mL. Change in PSA over time (PSA velocity), however, is not associated with AUR or surgery. As PSA is strongly correlated with prostate volume, it is not surprising that higher volumes (>30 to 40 mL by ultrasound) are associated with worsening LUTS and AUR. Other factors that increase AUR risk include previous episode(s) of retention, baseline postvoiding residual volume (PVR) greater than 100 mL, worsening LUTS, and failure to respond to alpha-blocker treatment.
Figure 50-4.
Natural history of LUTS. From a prospective study of 500 men, data available on 371 at 4 yrs. Baseline distribution of LUTS severity was mild 12%, moderate 49%, and severe 13%. (Symptom levels at (A) 1 yr and (B) 4 yrs. Bars indicate percent of men at follow-up with mild (white), moderate (gray), or severe (dark gray) LUTS or needing surgery (black). Data from Barry MJ, Fowler FJ Jr, Bin L, Pitts JC 3rd, Harris CJ, Mulley AG Jr. The natural history of patients with benign prostatic hyperplasia as diagnosed by North American urologists. J Urol 157:10–14, 1997.
Pathophysiology
Prostate hyperplasia occurs when prostate cell proliferation exceeds programmed cell death (apoptosis) as a result of stimulated cell growth, inhibition of apoptosis, or both. BPH occurs predominantly in the prostatic periurethral transitional zone, unlike prostate cancer, which tends to occur in more peripheral areas. BPH and prostate cancer are genetically distinct, and one is not a risk factor for the other.
The development of stromal and epithelial hyperplasia in BPH is androgen- and aging-dependent, and involves numerous paracrine and autocrine factors. The trophic androgen for prostate growth is dihydrotestosterone, produced from testosterone within the prostate by the enzyme 5α-reductase (thus the utility of 5α-reductase inhibitors for treatment). In the absence of 5α-reductase, even high serum levels of testosterone will not cause BPH. Additional factors supporting prostate cell growth include inflammatory cytokines (e.g., interleukin-8), autocrine cytokine growth factors, and neuroendocrine cell products. Stromal–epithelial interactions regulating proliferation also involve interactions between androgens, estrogens, and stimulatory and inhibitory peptide growth factors (e.g., fibroblast growth factor-2, transforming growth factor-β). Other regulatory factors include nitric oxide (nitric oxide synthetase levels are low in BPH), vitamin D, and local autonomic innervation and activity. Thus the development of BPH is the end result of numerous pathways involved in regulating sympathetic activity, androgen–estrogen balance, and smooth-muscle proliferation.
The divergence in prevalence between BPH, BPE, BOO, and LUTS results from many lower urinary tract factors (Table 50-1). In addition, numerous medical conditions, medications, and functional impairment may cause or worsen LUTS independent of prostate disease (see Chapter 59).
FACTOR | IMPACT |
|---|---|
Ratio of stromal to epithelial hyperplasia (stromal proliferation predominates in 50%, glandular in 25%, and mixed in 25%) | Stromal glands tend to be smaller, more symptomatic, and have worse symptomatic outcomes to prostatectomy |
Location of BPH nodules | Adenomas which occuring predominantly in central transitional and periurethral zones of the prostate, predispose to prostatic urethra compression. Adenomas in the median lobes may compress the bladder base without causing BOO. |
Fibroelastic properties of prostate capsule | Altered compliance may increase urethral compression in the absence of mechanical BOO. |
α-Adrenergic innervation of prostate | Increased number of α-adrenergic receptors promotes smooth-muscle contraction. |
Variability in detrusor muscle structure and function | Detrusor contractility can be affected by BPE/BOO-associated increased connective tissue infiltration; smoothmuscle hypertrophy and disintegration; decreased autonomic neuronal number; and conversion from predominantly β-adrenergic (inhibitory) to α-adrenergic (stimulatory) responsiveness. |
Detrusor overactivity | Detrusor overactivity (DO) is found in about two-thirds of men with BOO. Causality unclear as DO occurs in normal asymptomatic older men and women. DO resolves in up to two-thirds of men after TURP, yet tends to persist in the very elderly. |
Atherosclerotic disease | May cause detrusor ischemia leading to impaired contractility, and acute BOO from prostate infarction. |
Factors associated with the development of benign prostate disease include age, physical inactivity, high meat and fat intake (especially polyunsaturated fats), diabetes, high insulin levels, obesity, low high-density lipoprotein levels, and arteriovascular disease. Vasectomy is not a risk factor and the data on smoking are inconclusive.
Nocturia
Nocturia is defined as voiding at least once during the normal hours of sleep. As many as 80% of older men have nocturia, which is the most bothersome of all of the LUTS associated with benign prostate disease. The three main causes of nocturia are LUT pathophysiology, nocturnal polyuria, and sleep disturbance (Table 50-2). The latter two causes are especially important in older men because of the many age-related changes, comorbid conditions, and medications that can cause them.
|
Nocturnal polyuria is defined as the excretion of one-third or greater of the daily (24-hour) urine output during normal sleeping hours. Older persons are prone to nocturnal polyuria, possibly caused by higher nocturnal atrial natriuretic peptide levels and/or altered secretion of vasopressin. Some older person may excrete 50% or more of their 24-hour urine output during the night. For this reason, one episode of nocturia is considered normal in older persons and even the best treatment may not be able to completely eliminate nocturia. Another cause of nocturnal polyuria is peripheral edema (Table 50-2); edema fluid mobilizes when the patient reclines, leading to increased urine output. Sleep apnea is an underappreciated cause of nocturnal polyuria; apnea should be suspected when the patient or his partner report loud snoring, apneic periods, excessive daytime sleepiness, and morning headaches, and in men who are obese and/or have hypertension.
When aroused owing to primary sleep disorders, patients may sense their bladder volume (which tends to be higher at night) and get up and void, and report these episodes as “nocturia.” Common causes of sleep disturbance in older persons include age-related changes in sleep architecture (see Chapter 55); pain (e.g., from arthritis), depression, restless leg syndrome, gastric reflux, pulmonary and cardiac diseases, dementia, and the effects of many medications.
Evaluation
Benign prostate disease maybe asymptomatic, but most commonly presents with LUTS; other symptoms include urinary retention, recurrent urinary tract infections, or hematuria (from prostatic varices). Table 50-3 lists the recommended evaluation in men presenting with LUTS suggestive of benign prostate disease from the American Urological Association (AUA) 2006 Guideline on the Management of Benign Prostatic Hyperplasia. The sections below highlight aspects of the evaluation especially relevant to older men.
Recommended Evaluation of LUTS Suggestive of BPH
|
Optional Components
|
The evaluation of older men with LUTS closely parallels that of older persons with urinary incontinence (UI; see Chapter 59). The history should include the onset, progression, and associated factors for the common LUTS (slow stream, urgency, nocturia, etc.), and quantification of LUTS using the AUA Symptom Index (see below). Distinguishing between “irritative” and “obstructive” LUTS is not useful, because the terms are poorly specific and does not correlate with symptom bother, severity, or physiologic measures. Older men always should be evaluated for causes of LUTS other than prostate disease. The history and review of systems should question patients about hematuria, dysuria, and pelvic pain (rare in benign prostate disease, more suggestive of infection, bladder stone, prostate or bladder cancers); episodes of urinary retention; cardiac symptoms (regarding possible congestive heart failure in patients with nocturia); bowel and sexual function; type and amount of fluid intake (in relationship to frequency and nocturia symptoms); and sleep disturbance. All medications (including nonprescription drugs) must be reviewed, with a focus on drugs that can decrease detrusor contractility (anticholinergics, calcium channel-blockers), diuretics, drugs that can cause pedal edema (see Table 50-2), and α-adrenergic agents.
The American Urological Association Symptom Index (AUASI) quantifies the severity of BPH-associated LUTS on a scale 0 to 35; scores of 0 to 7 indicate mild symptoms, 8 to 19 moderate, and 20 to 35 severe (Table 50-4). An additional question, not tallied in the total score, assesses disease-specific quality of life impact. The similar International Prostate Symptom Score (IPSS) is widely translated and identically scored. A change in AUASI of ± 5 points has an 80% probability of indicating a true clinical change. The threshold for clinical change varies with symptoms severity (minimum perceptible difference is 2 points for AUASI <20 and 6 points for AUASI ≥20). Any magnitude of change, however, may be caused by interval changes in comorbidity and medications and not prostate disease.
1. Incomplete emptying: Over the past month, how often have you had the sensation of not emptying your bladder completely after you finished urination? | ||||||
Not at all | Less than 1 time in 5 | Less than half of the time | About half of the time | More than half of the time | Almost always | Your score |
0 | 1 | 2 | 3 | 4 | 5 | |
2. Frequency: Over the past month, how often have you had to urinate again less than 2 hours after you finished urinating? | ||||||
Not at all | Less than 1 time in 5 | Less than half of the time | About half of the time | More than half of the time | Almost always | Your score |
0 | 1 | 2 | 3 | 4 | 5 | |
3. Intermittency: Over the past month, how often have you found that you stopped and started again several times when you urinated? | ||||||
Not at all | Less than 1 time in 5 | Less than half of the time | About half of the time | More than half of the time | Almost always | Your score |
0 | 1 | 2 | 3 | 4 | 5 | |
4. Urgency: Over the past month, how often have you found it difficult to postpone urination? | ||||||
Not at all | Less than 1 time in 5 | Less than half of the time | About half of the time | More than half of the time | Almost always | Your score |
0 | 1 | 2 | 3 | 4 | 5 | |
5. Weak stream: Over the past month, how often have you had a weak stream? | ||||||
Not at all | Less than 1 time in 5 | Less than half of the time | About half of the time | More than half of the time | Almost always | Your score |
0 | 1 | 2 | 3 | 4 | 5 | |
6. Straining: Over the past month, how often have you had to push or strain to begin urination? | ||||||
Not at all | Less than 1 time in 5 |
|||||