Fig. 4.1
Endoscopic image of short-segment Barrett’s esophagus without nodularity. Prague C1M2
Several types of columnar epithelium can be found in the biopsy samples of suspected BE including gastric-fundic type, cardiac type, and intestinal type [10]. Gastric-fundic type is typically considered as part of a hiatal hernia and not thought to be associated with an increased risk of malignancy. There is controversy regarding whether a pathologic diagnosis of BE requires the presence of goblet cells within the BE segment (i.e., specialized intestinal metaplasia). In the USA, specialized intestinal metaplasia is considered the hallmark of BE as this is clearly associated with increased risk of malignancy [5, 6]. It is less clear whether there is a risk of malignancy associated with cardiac tissue that extends above the level of the GE junction. The most recent British guidelines propose cardiac-type epithelium be designated BE. These guidelines raise the concern that a lack of goblet cells may reflect sampling error and that non-goblet cell specialized intestinal metaplastic (SIM) epithelium may contain molecular features typically observed in goblet cell epithelium [7]. US societies, however, underscore the lack of definitive data supporting the concept that non-goblet cell SIM epithelium carries an increased risk of progressing to EAC, and note both the financial cost and negative emotional impact of placing patients with non-goblet cell BE under surveillance for a condition of unclear significance [5, 11–13].
BE has historically been divided into long-segment BE (>3 cm) and short-segment BE (<3 cm), which has been used to stratify BE patients into high risk (long-segment BE) and low risk (short-segment BE) (Fig. 4.2a–d). While increasing length of BE is clearly associated with increased risk for malignant progression, there is a lack of consensus regarding what differentiates a short-segment Barrett’s esophagus (SSBE) from an irregular squamocolumnar junction. The identification of SSBE is clinically important because of a small increased risk of EAC even in people with SSBE [14]. In addition, there are no current guidelines regarding the number of biopsies required to establish an initial diagnosis of BE. Due to the very low risk of malignancy associated with SSBE and ongoing debate regarding the value of current BE surveillance strategies, it is controversial whether an irregular z-line should be biopsied with the intent of making a diagnosis of BE [7].
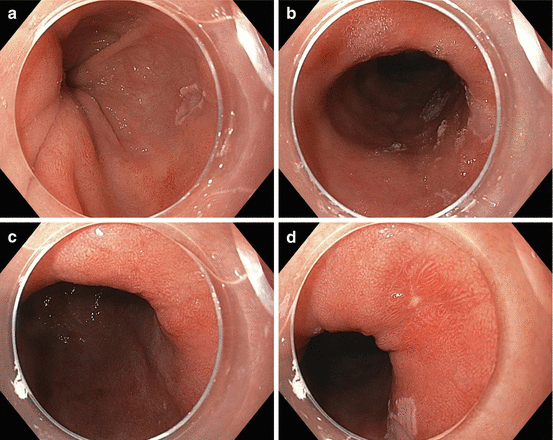

Fig. 4.2
(a–d) Endoscopic images of long-segment Barrett’s esophagus with low-grade dysplasia. Panels A–D show images from different individuals and reveal the range of appearance by endoscopy
Natural History and Risk Factors
Natural History
Norman Barrett was the first to describe the clinical finding of a columnar-lined epithelium that extended proximal to the gastroesophageal (GE) junction. The condition that bears his name is now recognized as the replacement of the normal esophageal squamous mucosa with a columnar-lined metaplastic epithelium [15]. Metaplasia is thought to arise as a response to esophageal reflux of hydrochloric acid and bile acids that damage the esophageal mucosa [16]. The subsequent progression of BE to dysplasia and eventually EAC is felt to involve the accumulation of a series of genetic and epigenetic alterations that are also likely driven in part by genotoxic damage secondary to reflux of gastric fluids [17]. As with other premalignant conditions such as colonic tubular adenomas, Barrett’s esophagus is asymptomatic and clinically important only because of its malignant potential. As an identifiable precursor to cancer, BE is a logical target for screening and surveillance programs with the end goal of decreasing the morbidity and mortality associated with esophageal adenocarcinoma (EAC) through the prevention or detection and treatment of early stage EAC. Unfortunately the utility of these programs is hindered by our incomplete understanding of the epidemiology, biology, and natural history of BE and EAC. Although it is thought to take many years for BE to transform into EAC, it is not clear how long the BE to EAC sequence takes. Nonetheless, data from patients in surveillance programs suggest that when high-grade dysplasia arises, there is often concurrent EAC, or the likelihood of progression to EAC is high over the next 6 months to 2 years [11, 18–20]. It also appears unlikely that BE spontaneously regresses once it has formed.
Risk Factors for BE and EAC
Risk Factors for BE and Strategies to Identify People to Be Screened for BE
The true prevalence of BE is unclear; however, estimates range from 1 to 18 %. The marked variability in prevalence estimates is presumably secondary to the variability of the underlying populations studied. In studies of patients referred for endoscopic evaluation of reflux symptoms or dyspepsia, BE can be found in between 6 and 18 % of patients, with higher percentages reflective of more stringent criteria for symptomatic reflux disease [21–23]. In US studies of patients referred for screening colonoscopy that additionally underwent upper endoscopy, BE was found in 6–8 % of patients [24, 25]. In the two largest population-based studies from Sweden and Italy, the incidence appears to be closer to 1–2 % [26, 27].
While gastroesophageal reflux disease (GERD) is thought to be the major mechanism mediating the formation of BE and chronic symptomatic GERD is the major clinical indication for screening for BE, reflux symptoms have poor sensitivity and specificity for identifying patients with BE. In some of the larger population studies noted above, less than 50 % of those with BE reported reflux symptoms [26, 27]. In other studies, those with symptomatic reflux disease and erosive changes on endoscopy were found to have a fivefold increased risk of developing BE in the following 5 years when compared to patients with nonerosive reflux disease [28]. In addition, symptomatic GERD may best predict those with long-segment BE as opposed to those with short-segment BE [29]. Notably, the longer reflux symptoms have been present, the greater the relative risk of BE [21]. However, the low prevalence of BE even in the setting of chronic GERD does not support the use of chronic reflux symptoms alone as an indication for BE screening [5, 7, 28, 30].
Aside from chronic gastroesophageal reflux, several demographic features are associated with an increased risk for intestinal metaplasia and dysplasia of the esophagus. BE has a higher incidence in Caucasians, males, and those with central adiposity. The incidence of BE also increases with age [31, 32]. With few exceptions, BE is rarely found in the young (in men below age 20, in women below age 40), African Americans, Asians, or women [31]. Other associated clinical risk factors include the presence of a hiatal hernia [21], obstructive sleep apnea [33], possibly diets low in fruit, and cigarette smoking [34]. The contribution of H. pylori remains unclear; however, there is some evidence that gastric infection with H. pylori may decrease the risk of developing BE [35], possibly through gastric mucosal atrophy and decreased parietal cell hydrochloric acid production.
Risk Factors for the Progression of BE to EAC
Once it develops, the time course for progression of BE to EAC is variable. Currently, the main recognized clinical risk factor for progression to adenocarcinoma is the degree of dysplasia present in BE. In the absence of dysplasia, large population studies suggest that the annual risk of progression to EAC is 0.12–0.40 % [11, 36, 37]. Those with low-grade dysplasia (LGD) have an annual risk of progression from 0.6 to 13.4 % [11, 18, 19, 38–40]. Studies are markedly divergent in the estimate of the annual risk of high-grade dysplasia progression, with the lowest estimates being 2 %/year and the highest reported risk at 59 %/year [41–45].
Among those diagnosed with BE, several risk factors are clearly associated with an increased risk for progression to EAC. In the largest reported population study, de Jonge assessed 42,207 Dutch patients with BE. EAC was found to be most closely associated with age >75 (hazard ratio (HR) 12), male sex (HR 2.01), and presence of LGD at the time of the baseline exam (HR 1.91) [37]. Additionally, a longer duration of heartburn symptoms and increased frequency of reflux symptoms were associated with a fivefold increased risk for the development of EAC [46].
Histologic stability of BE over time also associates with a decreased risk of progression of BE to EAC. In a multicenter cohort of patients with BE, those who had persistent non-dysplastic BE were least likely to develop EAC or progress to HGD at a median of 5 years of follow-up [47]. Prevalent cases are more likely to progress than incident cases. In a study of a series of patients with BE from Cleveland (N = 299), in patients with BE and LGD or indefinite for dysplasia (IND), the annual incidence rate of HGD or EAC was 2.4 % and 0.6 %, respectively. Within this group, the greatest risk for progression to HGD or EAC was for male patients, those patients with longer length of BE, and those patients with multifocal dysplasia and nodules seen on endoscopy. The median time after diagnosis of BE to diagnosis of HGD was 63.1 months and for EAC was 53 months. In this group, 58.5 % regressed to non-dysplastic BE (NDBE). Notably, prevalent cases of BE (those within 1 year of their BE diagnosis) had an increased risk of progression to EAC compared to incident cases [40]. These findings suggest that those at the highest risk for dysplastic progression devolve to cancer quickly and those with stable dysplastic changes over a course of years are less likely to progress. This may also explain why in a trial of radiofrequency ablation for dysplasia in BE, 19 % of patients with high-grade dysplasia developed cancer at 1 year [48]. As with regression, this may partially represent under-sampling or inter-observer pathologic disagreement; however, it may also represent that a more dynamic phenotype exists that progresses quickly to cancer in patients with more histologically advanced BE.
Just as there is variability in the likelihood of progression of BE, spontaneous regression of BE to squamous epithelium may occur but appears to do so variably and very infrequently. Some of the studies mentioned above suggest that as many as half of the patients diagnosed with LGD may regress to non-dysplastic BE [40, 49]. However, due to the known limitations of endoscopic surveillance (e.g., variability in the areas of BE biopsied from endoscopic exam to exam, small proportion of BE sampled, etc.) and modest consistency of the pathologic assessment of dysplasia (see further discussion below), it should be noted that some of the reported cases of regression may in fact be due to inter-observer pathologic disagreement or sampling error (i.e., areas of dysplasia not biopsied on follow-up exams). Features associated with regression are short-segment BE [40] and acid suppression with proton-pump inhibitor (PPI) medications [50–52].
Tissue-Based Diagnostic and Risk Markers
Broadly speaking, a biomarker is a detectable indicator for the presence or future risk of disease. In the case of Barrett’s esophagus, this term encompasses demographic measurements, such as BMI or smoking status, histologic findings such as low-grade dysplasia (LGD) or inflammation, and molecular markers such as aneuploidy or tetraploidy or aberrant DNA methylation [53]. Symptoms of long-standing gastroesophageal reflux disease (GERD) have historically been the main clinical features used to identify people at risk of having BE, who are then advised to undergo endoscopic assessment. It is clear that many people with BE have no history of GERD, which is one of the reasons behind the lack of clear success of current BE surveillance programs for preventing EAC [17]. With regard to strategies to identify BE at high risk of progressing to EAC, the presence or absence of BE with dysplasia on histologic review is currently the only biomarker used clinically for risk stratification and directing treatment [5–7]. This dearth of well-studied biomarkers and the reliance on reflux symptoms and endoscopic findings of dysplasia had led to what Reid has called the “paradox” of BE management. In this paradox, Reid notes several frustrating epidemiologic facts: (1) a large number of individuals with BE are asymptomatic, (2) nearly 50 % develop EAC without associated GERD symptoms, (3) 95 % of EAC arise without a prior diagnosis of BE, and (4) nearly 80 % of EAC arise without a prior diagnosis of GERD [17]. The vast number of people with BE detected by endoscopy will not progress to EAC and instead will die of unrelated causes. In fact, the majority of people with BE are more likely to die from complications of cardiac disease than from EAC [54]. With these insights, several areas of active research in molecular biology are underway to resolve the “paradox” of BE and may lead to a more effective approach to identifying and managing those patients with BE. A number of promising markers have been identified; however, currently there are only a limited number of biomarkers available to precisely identify patients with BE and BE patients at high risk of progression to EAC. We will next discuss the most promising markers currently under investigation.
With recent advances in genomics (i.e., next-generation sequencing), epigenomics, proteomics, and microarray technology, many promising diagnostic and prognostic molecular biomarkers have been identified at the level of DNA, RNA, and individual proteins. Examples of types of prospectively tested molecular biomarkers include chromosomal alterations such as abnormal DNA ploidy and alterations in DNA copy number (based on fluorescence in situ hybridization (FISH)) [55–57], gene mutations, aberrantly methylated genes, loss of heterozygosity of specific DNA loci [49], and measurements of clonal diversity in the BE tissue [58]. These molecular alterations may serve as adjunctive markers to delineate the degree of dysplasia (e.g., use of FISH probes for C-MYC to confirm high-grade dysplasia or carcinoma [57]) or to further risk stratify patients at the greatest risk for progression to EAC (e.g., loss of aneuploidy or tetraploidy associates with a 38.7 % increased relative risk of developing EAC [55]). Other non-prospectively evaluated but promising markers include epigenetic alterations in the form of aberrantly methylated gene panels [59] and alterations in gene mRNA and microRNA expression [60]. Several groups have also explored the use of protein markers to augment endoscopic visualization and diagnosis of BE with dysplasia [56]. At this time, these studies are limited by a lack of suitably large prospective clinical validation trials because of the lack of sizeable esophageal tissue repositories from appropriate patient populations [59].
A majority of the currently reported biomarkers require selective endoscopic biopsies. Non-endoscopic methods for acquiring BE tissue samples that are currently being evaluated experimentally include the Cytosponge™, a swallowed capsule that degrades in the stomach to release a sponge tethered to a string. As the sponge is pulled back through the esophagus and out of the mouth, it captures esophageal cells which can later be analyzed for particular molecular changes associated with BE and/or dysplasia [2]. In a clinic-based study of British patients with reflux disease, researchers assessed the feasibility of an assay that measured trefoil factor 3 (TFF3) expression in esophageal samples collected with the Cytosponge™. The TFF3 assay had a reasonable sensitivity (90 %) and specificity (93.5 %) for segments of BE greater than 2 cm in length. The authors reported the Cytosponge™ was well tolerated with minimal anxiety or discomfort during its use [61]. Subsequent cost-effectiveness modeling studies demonstrated the use of the Cytosponge™ for BE screening to be cost-effective with the potential to reduce mortality from EAC as compared to a no-screening strategy [62].
BE Surveillance: Current Clinical Strategies and Features That Affect the Implementation of the Surveillance Program
Once the diagnosis of BE is established, an appropriate assessment should be performed to stratify the patient into a low- or high-risk group for the development of esophageal cancer. Risk-stratification dictates specific aspects of the recommended BE surveillance program. The index endoscopy description should include information on the length of the esophagus involved, the presence or absence of nodularity or other lesions, and whether esophagitis or atypical appearing glands were present [5, 7].
Because the length of BE directly correlates with increasing risk of malignancy, it is recommended that the endoscopist clearly documents the length of the visualized BE segment as well as the circumferential extent [5–7]. In order to improve the consistency of the description of these endoscopic features, there is multi-society support for the use of the “Prague classification” to describe BE. This system mandates that the endoscopist reports the length of circumferential involvement and the maximal length of BE measured from the GE junction (e.g., 4 cm of circumferential involvement with a tongue extending an additional 2 cm would be reported as C4M6). Standardization of the description of BE using the Prague classification promises to both facilitate communication between providers and to assist the endoscopist in determining response to therapy. There is reasonable agreement between operators using this classification tool [63, 64].
Another critical feature that governs specific aspects of a BE surveillance program is the presence of dysplasia. It is important to recognize that the presence of esophagitis, either visualized on endoscopy or by histology, impairs the ability of the pathologist to diagnose dysplasia and may result in a pathologic description of “indefinite for dysplasia (IND)” [7]. In this instance, the patient is advised to comply with aggressive anti-GERD therapy (i.e., twice daily proton-pump inhibitor (PPI) therapy) for at least 3 months and then have follow-up endoscopic biopsies performed. It is also important to recognize that esophageal ulceration may represent invasive EAC when high-grade dysplasia (HGD) is present [65].
The presence of mucosal abnormalities, particularly nodularity within a segment of BE, is concerning for dysplasia if not more advanced neoplasia, particularly in the absence of esophagitis [66–68]. If nodularity is visualized on endoscopy, standard practice is for endoscopic mucosal resection (EMR) of the affected segment [5, 7] (The technique of EMR is discussed below.). For staging, EMR provides more tissue than can be obtained with standard endoscopic biopsies. Consequently, EMR has proved more useful than adjunctive staging techniques such as CT or endoscopic ultrasound for distinguishing HGD from EAC. The use of EMR improves the certainty of pathologic diagnosis and staging of superficial EAC lesions, in part by increased inter-observer agreement and better diagnostic reproducibility when compared to standard biopsy specimens [69–73].
The use of high-definition white light endoscopy (HD-WLE) theoretically improves the endoscopist’s ability to identify BE and to identify abnormal and potentially dysplastic glands within the segment of BE, though studies demonstrating the superiority of HD-WLE over conventional “low-resolution” endoscopy are lacking. At the present time, HD-WLE remains the standard of care for the endoscopic management of BE. More recently developed technologies such as narrow band imaging (NBI), confocal microscopy, chromoendoscopy, and optical coherence technology are still being assessed with regard to their role in the management of BE and are not currently recommended for generalized use [5, 7].
The Role of Dysplasia in Risk Stratification and in Guiding BE Surveillance and Treatment
Dysplasia is a central factor in determining the risk for EAC and for guiding a BE surveillance program. The Vienna classification system divides BE into five categories: no dysplasia, indefinite for dysplasia, low-grade dysplasia, high-grade dysplasia, and invasive neoplasia [74]. Experts hoped that this classification would lead to a more unified terminology between pathologists. Notably, the transformation of BE from LGD to HGD and then to intramucosal carcinoma (IMC) occurs along a spectrum. As such, it is important to note two points: (1) the artificial separation between LGD, HGD, and IMC results in difficulties with inter-observer variability and (2) a single biopsy specimen only represents a small minority of the larger field of BE. Because much of the BE management algorithm rests on differentiating subtypes of dysplasia, societal guidelines recommend that two expert pathologists review biopsies reported as dysplastic or intramucosal carcinoma [5–7]. A challenge in managing BE is the known lack of intra- and interobserver agreement for pathologic assessment of the degree of dysplasia. This lack of agreement holds true even between expert pathologists [19, 74–76]. This lack of agreement is thought in part due to relatively arbitrary discrete categories placed on the continuum of dysplastic BE, and while there is relative consensus for non-dysplastic BE and for IMC, separating LGD and HGD remains challenging [77]. Despite this limitation, the degree of dysplasia remains the only clearly identified biomarker which has received support across clinical societies for directing specific treatment regimens [5–7].
The variability in the reported risk of progression based on dysplasia is relatively striking. It is clear that those with non-dysplastic BE have a relatively low risk of adenocarcinoma; however, the reported range of risk of progression to EAC for dysplastic BE is marked [16]. One of the explanations given for this variability is the reliance on forceps biopsy. Within a column or tongue of Barrett’s, there may be several areas of dysplastic foci, and these areas can be easily missed using standard biopsy forceps. The commonly accepted “Seattle Protocol” relies upon four-quadrant biopsies every 2 cm in non-dysplastic BE or every 1 cm in dysplastic BE. However, even when this aggressive tissue sampling protocol is performed appropriately, small foci of dysplasia may be missed [11].
Treatment of Barrett’s Esophagus
The treatment of BE is primarily focused on the prevention of BE progressing to EAC. Until recently, this meant that the focus of BE treatment was on managing risk factors for progression, such as controlling acid reflux, and on endoscopic surveillance to detect HGD or carcinoma, at which point patients were referred for surgical resection of the esophagus. Despite improvements in minimally invasive surgical techniques, the morbidity and mortality of esophagectomy remain high (2–5 %) even at expert centers [78]. This high mortality prompted the development of endoscopic treatments for BE. These modalities include ablative techniques such as radiofrequency ablation (RFA), cryotherapy, photodynamic therapy (PDT), and argon plasma coagulation (APC) and resective therapies such as endoscopic mucosal resection. While surgical management remains the standard of care for patients with esophageal cancer that has progressed beyond the level of IMC, endoscopic treatments are increasingly used and accepted for patients with dysplastic BE [5–7].
Non-endoscopic Management of BE
Lifestyle Modifications
The only currently recommended non-endoscopic management strategy for BE is the avoidance of risk factors known to be associated with the risk of BE progression to EAC. Based on the risk factors described above, it is intuitive, albeit poorly studied, that avoiding smoking, decreasing central adiposity, and consuming a diet high in fruits and vegetables should be recommended to all patients with a diagnosis of BE. Less intuitive is the recommendation that patients with BE have an assessment of their cardiovascular function and/or cardiovascular disease risk factors. In a population-based study from the UK, individuals diagnosed with BE had an increased risk of death from esophageal cancer; however, the largest single cause of death was ischemic heart disease. BE patients had a fourfold increased risk of dying from ischemic heart disease versus esophageal cancer [54]. This raises the interesting question of whether patients diagnosed with BE would be better served by first optimizing their cardiovascular health before embarking on extensive screening and surveillance programs for their BE.
Chemoprevention
There is no proven agent that is effective for primary or secondary chemoprophylaxis for Barrett’s esophagus. Even though there is considerable data in preclinical models that reflux of acid/bile is a central mechanism for BE formation and progression, there remains no evidence that patients with asymptomatic reflux have a mortality benefit from the use of proton-pump inhibitors (PPIs) or histamine 2 receptor inhibitors [5, 7]. Nonetheless, the use of PPI medications irrespective of symptomatic reflux has been recommended by some authorities [79] although most society position statements do not promote PPI use in the absence of symptomatic reflux. There is relatively strong support for the use of PPIs in BE patients with symptomatic GERD or those patients undergoing endoscopic eradication therapy [5, 7].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree