Fig. 52.1
Roux-en-Y gastric bypass (RYGB) surgery. Diagram courtesy of Dr. Daniel Herron
Optimizing Glycemic Control Prior to Surgery
An appropriate preoperative screening includes prior history of failure to lose weight despite proper medical therapy. Patients must be educated regarding the bariatric procedure and the need for lifelong adherence to nutritional management. Glycemic control must be optimized in patients with diabetes prior to surgery in order to minimize the perioperative complications. Preoperatively, patients are more motivated to optimize their medical condition and endocrinologists can help manage their diabetes aggressively. Starting an insulin regimen is appropriate in order to optimize the glycemic control even in a relatively short period of time for patients preparing for the surgery. Perioperative tight glycemic control often requires some insulin coverage, but it must be adjusted accordingly to their insulin requirement (see discussion 2 below). Successful outcome after a surgical weight loss procedure depends on extensive patient counseling and multidisciplinary support postoperatively.
Long-Term Outcomes of Bariatric Surgery
Bariatric surgery appears to be an effective option for the treatment of severe obesity, resulting in a long-term weight loss, an improved lifestyle, and, an amelioration in risk factors associated with obesity (Fig. 52.2).
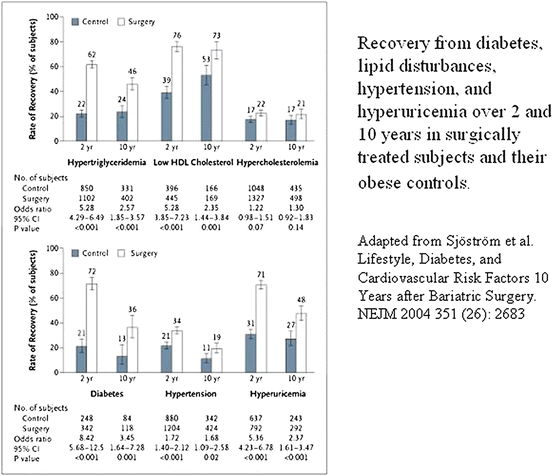

Fig. 52.2
Recovery from diabetes, lipid disturbances, hypertension, and hyperuricemia over 2 and 10 years in surgically treated subjects and their obese controls. Adapted from Sjöström et al. Lifestyle, Diabetes, and Cardiovascular Risk Factors 10 years After Bariatric Surgery. NEJM 2004; 351 (26): 2683
The prospective controlled Swedish Obese Subjects Study involved obese subjects who underwent gastric surgery and contemporaneously matched, conventionally treated obese control subjects. One of the largest series, with 4,047 subjects, it demonstrates the benefit of bariatric surgery for morbid obesity [2]. Significant number of patients with diabetes can recover with surgical weight loss, based on either the cutoff values or use of medication to treat diabetes. The mean changes in weight and risk factors were also more favorable among the subjects treated by gastric bypass than among those treated by banding or other form of surgical procedures.
Diabetes Resolution with RYGB Surgery
The duration of diabetes since diagnosis seems to predict successful improvement of glycemic control postsurgery. The first phase insulin response is typically disrupted early in the course of diabetes and it improves to a near-normal level in patients who undergo gastric bypass surgery with diabetes of less than 3–5 years duration [3]. Recovery of the first-phase insulin response may be the best indicator of diabetes resolution in patients who have had gastric bypass surgery (Fig. 52.3).
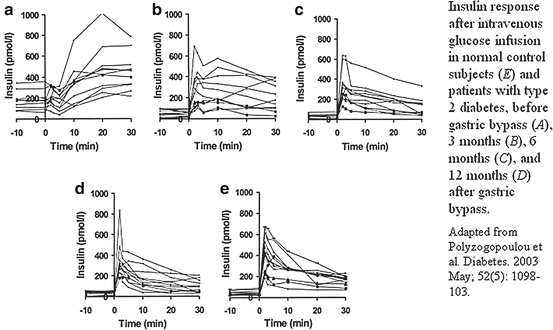

Fig. 52.3
Insulin response after intravenous glucose infusion in normal control subjects (e) and patients with type 2 diabetes , before gastric bypass (a), 3 months (b), 6 months (c), and 12 months (d) after gastric bypass. Adapted from Polyzogopoulou et al. Diabetes. 2003 May; 52(5): 1098–103
The mechanisms underlying the effects of RYGB on body weight and glucose metabolism are still not completely understood, but we can predict the course of diabetes outcome with surgery. In the immediate postoperative period, patients are essentially fasting and resulting in the fast-induced alleviation of diabetes. Patients who are on significant doses of insulin preoperatively often only require a minimal basal insulin. Patients gradually tolerate their oral intake, but they continue to be in a state of negative energy balance, a condition that decreases glucose toxicity and improves ß-cell function. Eventually, a marked weight reduction with baritaric surgery allows patients to increase their level of physical activity. Increased physical activity coupled with decreased glucose load from small quantity of each meal after gastric bypass surgery leads to a dramatically improved diabetes control.
Incretin Effects of RYGB Surgery
Cummings hypothesizes more interesting possibilities of RYGB effect on glucose metabolism [4]. Alterations in gut hormones release after RYGB may act in concert with the above mechanism to improve insulin secretion or action. Ghrelin, secreted by the stomach, exerts several diabetogenic effects including increased levels of GH, cortisol, and epinephrine—three of the four classical counter regulatory hormones. Ghrelin levels are decreased after gastric bypass, resulting in an antidiabetogenic effect. The other effect of surgery is increased GLP-1 secretion from bypassing part of the foregut and facilitated delivery of nutrients directly to the hindgut. GLP-1 is an incretin that stimulates insulin secretion in response to enteral nutrients. GLP-1 and PYY also suppress gastrointestinal motility, gastric emptying, small intestinal transit, and food intake.
Patients with a relatively short duration of diabetes since diagnosis seem to have a better improvement of glycemic control post-RYGB [6]. While there are no standardized guidelines for the optimal timing to recommend patient with diabetes and obesity for bariatric surgery, these findings may call for an earlier referral of morbidly obese patients with diabetes to a surgical weight loss (Table 52.1).
Table 52.1
Preoperative predictors of improvement in type-2 diabetes
Factors | Improvement | Resolution |
|---|---|---|
Duration of T2 DM | <10 years
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|