Atypical Adenomatous Hyperplasia
Keith M. Kerr
This chapter considers the current theories of adenocarcinogenesis in the human lung and the place that atypical adenomatous hyperplasia (AAH) has in this process. AAH is a putative precursor of lung adenocarcinoma and recognized in the WHO classification of lung tumors as a preinvasive lesion.1 It is now believed that at least a proportion of lung adenocarcinomas, most of which arise in the peripheral parenchymal compartment of the lung, develop through what has been referred to as an adenoma-carcinoma sequence. In making this comparison with adenocarcinogenesis in the colon, AAH is the equivalent of the adenoma. Indeed, when the late Dr. Roberta Miller of Vancouver made this comparison between the colon and the lung, she referred to AAH lesions as bronchioloalveolar adenomas.2,3 The term AAH has, however, emerged from a number of different synonyms as the preferred term.
With the benefit of hindsight there are descriptions of lesions we would now consider AAH in work published in the 1950s and 1960s, but the first clear description of AAH with a suggestion of the importance of this lesion in adenocarcinogenesis was made by Shimosato et al.4 in 1982. The lesions were referred to as “atypical alveolar cuboidal cell hyperplasia” and Shimosato speculated that “one can assume that some peripheral adenocarcinomas arise without any association with preexisting scar tissue as an in-situ carcinoma from almost normal appearing bronchioloalveoli. However, it is not certain whether or not cancer develops through a stage of atypical hyperplasia.” Several years elapsed before a few more publications appeared on this subject, including Roberta Miller’s two seminal papers in 1988 and 19902,3: the vast majority of the literature on AAH, however, emanates from Japan. Shimosato et al.4 and Noguchi et al.5 deserve credit for formulating the hypothesis of a step-wise progression of disease in the lung periphery whereby AAH lesions transform into a lesion currently known as localized nonmucinous bronchioloalveolar carcinoma (LNMBAC), a lesion that could better be referred to as adenocarcinoma in situ. LNMBAC lesions tend to undergo alveolar collapse and fibroelastosis. At some point invasion supervenes, usually in the center of the lesion, and invasive adenocarcinoma is defined.
The epithelial compartment of the peripheral lung is functionally diverse and is regarded quite separately from central bronchial epithelium. Peripheral epithelium has a different population of stem cells from those in the bronchus. There are a number of stem cell candidates, most of which reside in the peripheral bronchiolar epithelium which, with alveolar epithelium, makes up the peripheral bronchioloalveolar epithelial compartment. Some differentiated cells appear to have stem-like properties.6,7 A variant of the Clara cell which shares an immunophenotype with alveolar pneumocytes and is found at the bronchioloalveolar duct junction is one such putative stem cell. Cells with neuroendocrine characteristics, found in bronchiolar neuroendocrine bodies, also appear to behave like stem cells. Not much is known about the biology of these particular cells but it seems likely that they, perhaps especially the variant Clara cells, are the population that gives rise to AAH. This concept fits neatly with the terminology, proposed by Yatabe et al.,8,9 of terminal respiratory unit (TRU) carcinogenesis and TRU-type adenocarcinomas.
Adenocarcinoma has replaced squamous cell carcinoma as the most prevalent form of lung cancer worldwide. For many years this was the case in many East Asian countries but it is a more recent development in North America and at least some European countries. In central and
eastern Europe, squamous cell carcinoma still appears to dominate. Although the association between tobacco smoking and adenocarcinoma is less strong than it is with squamous or small cell carcinoma, there is a dose-response relationship between smoking and risk of developing adenocarcinoma. While it is true that most lung cancers encountered in nonsmokers are adenocarcinomas, most adenocarcinomas, at least in European and North American populations, are found in smokers. The reasons for this rise in adenocarcinoma are not clear but a number of factors may be relevant. Changes in cigarette manufacture have led to lower nicotine and tar levels in smoke but also a change in many other carcinogens, including a rise in N-nitrosamines such as the potent carcinogen NNK (see also Chapter 26). There is some evidence that NNK and other nitrosamines may be more carcinogenic to the TRU and give rise to more adenocarcinomas.10,11 Smoking habits have changed. Lower nicotine and tar levels and other alterations to the “smoking experience” such as filters mean that smokers of the “modern cigarette” inhale more deeply than earlier consumers. This, it has been suggested, exposes the peripheral lung to more carcinogens. In many countries the proportion of women who smoke has risen. This accounts for the observed rise in female lung cancer. Women seem more prone to develop adenocarcinoma but whether this is because the female smoking generation is consuming a more “adenocarcinogenic” cigarette than their male predecessors is not clear. Adenocarcinoma has also risen in males, suggesting the cigarette may be the culprit. As is discussed below, however, women seem more prone to develop AAH. The relationship between AAH and smoking is not known; are AAH more likely to develop in female rather than male smokers or are females more likely than males to develop AAH for some other reason, and AAH then becomes transformed into LNMBAC and adenocarcinoma?
eastern Europe, squamous cell carcinoma still appears to dominate. Although the association between tobacco smoking and adenocarcinoma is less strong than it is with squamous or small cell carcinoma, there is a dose-response relationship between smoking and risk of developing adenocarcinoma. While it is true that most lung cancers encountered in nonsmokers are adenocarcinomas, most adenocarcinomas, at least in European and North American populations, are found in smokers. The reasons for this rise in adenocarcinoma are not clear but a number of factors may be relevant. Changes in cigarette manufacture have led to lower nicotine and tar levels in smoke but also a change in many other carcinogens, including a rise in N-nitrosamines such as the potent carcinogen NNK (see also Chapter 26). There is some evidence that NNK and other nitrosamines may be more carcinogenic to the TRU and give rise to more adenocarcinomas.10,11 Smoking habits have changed. Lower nicotine and tar levels and other alterations to the “smoking experience” such as filters mean that smokers of the “modern cigarette” inhale more deeply than earlier consumers. This, it has been suggested, exposes the peripheral lung to more carcinogens. In many countries the proportion of women who smoke has risen. This accounts for the observed rise in female lung cancer. Women seem more prone to develop adenocarcinoma but whether this is because the female smoking generation is consuming a more “adenocarcinogenic” cigarette than their male predecessors is not clear. Adenocarcinoma has also risen in males, suggesting the cigarette may be the culprit. As is discussed below, however, women seem more prone to develop AAH. The relationship between AAH and smoking is not known; are AAH more likely to develop in female rather than male smokers or are females more likely than males to develop AAH for some other reason, and AAH then becomes transformed into LNMBAC and adenocarcinoma?
There have been no studies of the biochemical influences that smoking may have on AAH. A small number of studies have shown altered gene expression in morphologically normal peripheral lung epithelium in smokers. As with similar studies on bronchial epithelium (see Chapter 26), among the differentially expressed genes in the smokers studied were those coding for xenobiotic metabolizing enzymes involved in the detoxification but also activation of tobacco carcinogens, and genes involved in cell cycle regulation.12
Although the combined literature addressing the molecular biology of AAH is less than that concerning central bronchial carcinogenesis, some interesting facts are emerging which give insight into the probable molecular events driving peripheral lung adenocarcinogenesis. These are reviewed below. There is good evidence, however, that the stepwise morphological progression of disease from AAH to LNMBAC to invasive adenocarcinoma is accompanied by progressive accumulation of genetic changes. It has been suggested that between 3 and 12 critical genetic events must occur, in the correct order, during lung carcinogenesis.13 This issue and how it relates to field carcinogenesis in the central airway’s epithelial compartment is discussed in a little more detail in Chapter 2 but many of the principles are also relevant to TRU carcinogenesis. While some of the genetic events in the AAH-LNMBAC sequence are common to those seen in bronchial squamous dysplasia and carcinoma in situ (SD/CIS), there are also differences. As with SD/CIS, it is likely that the observed morphological progression of disease is a reflection of accumulation of genetic changes. The morphology of these lesions is discussed in the next section.
PREINVASIVE LESIONS OF THE PERIPHERAL LUNG
AAH is defined as a preinvasive lesion in the WHO classification of lung tumors as follows: “a localised proliferation of mild to moderately atypical cells lining involved alveoli and, sometimes, respiratory bronchioles, resulting in focal lesions in peripheral alveolated lung, usually <5 mm in diameter and generally in the absence of underlying interstitial inflammation and fibrosis.”1 The last part of the definition is important as it emphasizes one of the factors which helps distinguish this lesion from the reactive proliferation of bronchioloalveolar epithelium seen in a number of inflammatory and fibrosing conditions in the lung. Some of these latter pathological diseases do appear to be associated with an increased risk of developing lung cancer, together with other lung lesions that also appear to have the potential to undergo
malignant change. Reactive bronchioloalveolar cell hyperplasia is also an important differential diagnosis of AAH; this is considered below.
malignant change. Reactive bronchioloalveolar cell hyperplasia is also an important differential diagnosis of AAH; this is considered below.
Two relevant issues are raised in this thread of discussion. The first is the recognition that, as in the central airways, cell proliferation seems to be an important early step in the development of neoplasia in the lung periphery. The second is rather more semantic and concerns the distinction between hyperplasia, a reactive phenomenon, and neoplasia, also a “reactive” proliferation of sorts but one that has, by definition, become, to some extent, autonomous and independent of the original stimulus that triggered the “new growth”. Sometimes the distinction between these two processes is distinctly blurred; this is certainly the case with AAH.
AAH is the best recognized and most widely studied preinvasive lesion in the peripheral lung. Another lesion named bronchiolar columnar cell dysplasia (BCCD) has been proposed as an alternative preinvasive lesion arising in the TRU epithelial compartment. Descriptions of this lesion are few but seem to describe cytological atypia in bronchiolar epithelium. While this cannot be dismissed as a possible alternative precursor of lung cancer in the peripheral lung (one of the two papers describing this lesion considers BCCD, a potential precursor lesion, for peripheral squamous cell as well as adenocarcinoma), it is poorly recognized.14 Until there are more published studies on BCCD, and experience of the lesion is more widespread, it is difficult to come to any conclusion regarding this lesion’s place in the current discussion. The lesion will be more widely recognized only when clear criteria for diagnosis are established and accepted. One may occasionally identify what might be BCCD, but there is no data to relate it to the development of pulmonary carcinomas.
The evolution of cancer through a series of morphologically recognizable precursor stages has been referred to as the “sequential model” of carcinogenesis; examples would be SD/CIS as precursors of bronchial squamous cell carcinoma and AAH/LNMBAC (adenocarcinoma in situ) as precursors of adenocarcinoma. The “parallel model” of carcinogenesis considers the possibility that carcinoma may arise de novo from an epithelium without passing through a morphologically recognizable precursor stage.15 This has been proposed as a mechanism through which small cell lung carcinoma (SCLC) may arise.16 Although SCLC has been linked with SD/CIS, it is impossible to deny that SCLC may also arise in this rather more abrupt fashion, without a progenitor lesion; there is some molecular evidence to support this suggestion. Equally, it is possible that some lung adenocarcinomas may arise in this way, from the TRU epithelium or, indeed, from more central bronchial epithelium.
ATYPICAL ADENOMATOUS HYPERPLASIA, GROSS FEATURES
The identification of AAH is largely the preserve of pathologists. There is an emerging literature on the high resolution computed tomographic (HRCT) scanning appearances of AAH discovered in the course of lung cancer screening using this imaging modality. This work comes almost exclusively from Japanese and other East Asian centers.17 Reference to this work will be made below. Most AAH lesions are incidental findings during microscopic examination of the alveo-lated lung. A proportion of AAH lesions may be identified macroscopically but success in this search is highly dependent upon the absence of concurrent pathology in the lung parenchyma under examination and upon how well prepared the lung tissue is before and during sectioning.
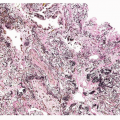
In well-prepared material, AAH lesions appear as poorly defined pale patches of a few millimeters in diameter on the cut surface of the lung parenchyma (Fig. 27-1). Their color ranges from gray through tan to cream or yellowish. The margins of the lesions are indistinct, blending into the surrounding lung. AAH lesions are rarely palpable on the lung cut surface. The surface of some lesions may be stippled by a few tiny pits that represent the alveolar spaces within the lesion. Occasional lesions may appear cystic; this is due to small foci of centriacinar emphysema arising in the same area as the AAH. These gross findings are not specific; the author finds that many lesions with this gross appearance are nonspecific inflammatory foci or tiny areas of fibrosis.
It should be clear from this description that the presence of other pathology such as pneumonia, extensive emphysema, or lung fibrosis will make the macroscopic identification of AAH more or less impossible.
It should be clear from this description that the presence of other pathology such as pneumonia, extensive emphysema, or lung fibrosis will make the macroscopic identification of AAH more or less impossible.
 FIGURE 27-1 Gross image of AAH lesion. The small holes in the lesion represent individual alveolar spaces. |
Adequate preparation of the lung tissue before gross inspection is required to maximize the chances of identifying AAH lesions, at gross inspection or during microscopy. Whenever possible, all lung resection specimens (lobectomy, pneumonectomy, wedge resections, etc.) received fresh from the operating room should be inflated with 10% neutral buffered formalin, either per-bronchially or by needle and syringe. After 24 hours suspended floating in fixative, the specimens are serially sectioned. Larger resection specimens are cut into 1-cm thick parasagittal slices using a specially designed cutting board. Lung slices are then laid out on the cutting bench and periodically flooded with clean water while being observed under a bright light. The water partially reexpands the lung parenchyma, making small lesions easier to identify. All possible AAH lesions are sampled; if none are seen, then three to six random parenchymal tissue blocks are taken. Methods of tissue preparation and sampling vary greatly and this may be one reason for the differences in reported prevalence of AAH.
ATYPICAL ADENOMATOUS HYPERPLASIA, HISTOLOGICAL FEATURES
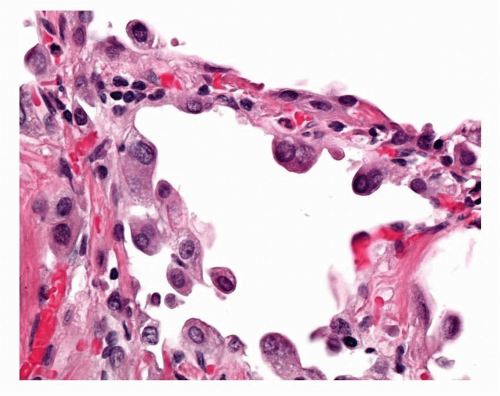
The WHO definition of AAH is given above. It is relevant to note that, given the earlier discussion of the possible role of BADJ stem cells in the evolution of TRU adenocarcinogenesis, these lesions appear to arise in a centriacinar location, the alveolar walls within and surrounding the respiratory bronchioles and alveolar ducts forming the framework for lesion growth (Fig. 27-2). The alveolar walls of an AAH lesion are lined by a single intermittent layer of cells that may be flattened, cuboidal, or low columnar; some cells appear round or globular, and others have apical snouts, sometimes referred to as hobnail or “peg cells” (Fig. 27-3). The cell population typically is quite heterogeneous and gaps between the larger cells which protrude into the alveolar spaces give the cell lining its “intermittent” character (Figs. 27-4 and 27-5). The cell cytoplasm is generally fairly nondescript; occasionally, small vacuoles may be seen. Ultrastructural and immunohistochemical studies have shown that the cells in AAH can show alveolar pneumocyte or Clara cell differentiation. Both may be found in the same lesion. Ciliated cells or mucous cells are not found in AAH.
Nuclei are generally regular with little pleomorphism. Nuclear inclusions are not uncommon in AAH. Nuclei can appear quite hyperchromatic and occasional large and irregular nuclei can be found. Some cells may show double nuclei, often located in the apical cytoplasm of an enlarged cell (Fig. 27-6). Mitotic figures are extremely uncommon.
 FIGURE 27-3 The cells lining the alveolar walls in AAH characteristically, in most lesions, form this interrupted lining with intercellular gaps. Some of the cells are quite large. |
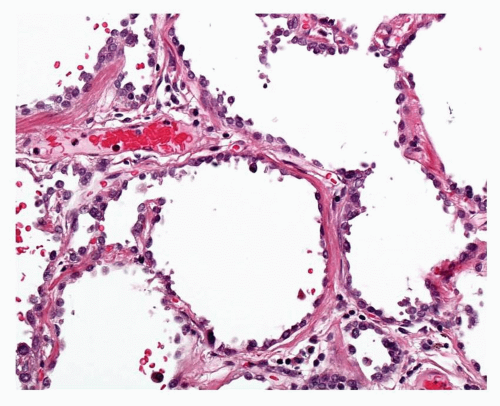 FIGURE 27-4 Higher power image of typical relatively low-grade AAH. The hobnail features of some of the cells can be seen. Some nuclei are apical as opposed to basally located. |
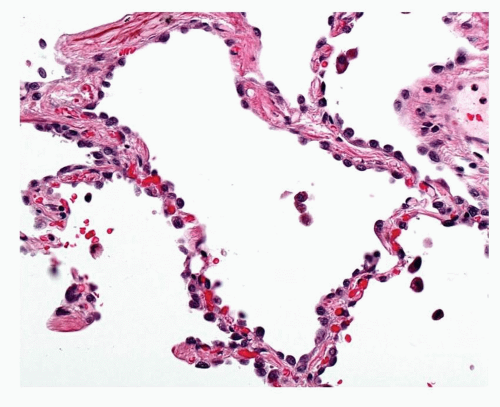 FIGURE 27-5 High-power image of low-grade AAH in Figure 27-4 showing hobnail cells and occasional cells show intranuclear inclusions. |
The alveolar walls within the AAH lesion are usually a little thickened due to an increase in collagen and also some elastosis. Occasional examples may be encountered where this process is more marked (Fig. 27-7). This may be associated with apparent shrinkage of the alveolar spaces or collapse of the alveolar architecture; this phenomenon is well described in LNMBAC but can also occur in AAH. The fibrotic interstitium may take on a rather hyalinized character. Chronic inflammatory cell infiltrates may be present. These are generally light in character but some lesions may be heavily infiltrated and lymphoid aggregates may be seen. This inflammatory process does not extend beyond the limits of the lesion as defined by the epithelial cell component, an important factor in distinguishing AAH from reactive hyperplasia in interstitial lung disease. Occasional lesions will be seen where only part of the lesion is fibrosed and the epithelial lining cells are lost. It is possible that this is a process that progressively leads to regression of the AAH lesion into a small hyalinized bronchioloalveolar scar.
AAH lesions are small localized foci of millimeter size, which usually stand out from the surrounding normal lung parenchyma due to the slight thickening of the alveolar walls and studding of the alveolar wall lining by enlarged epithelial cells. Lesions may also be highlighted by the
tendency for alveolar macrophages to accumulate within the alveolar spaces; this presumably, at least in part, is a result of the centriacinar location of AAH. Occasionally, in cases where there are very large numbers of AAH identified, larger AAH-like zones may be encountered where margins are difficult to discern, perhaps the result of merging of adjacent lesions.
tendency for alveolar macrophages to accumulate within the alveolar spaces; this presumably, at least in part, is a result of the centriacinar location of AAH. Occasionally, in cases where there are very large numbers of AAH identified, larger AAH-like zones may be encountered where margins are difficult to discern, perhaps the result of merging of adjacent lesions.
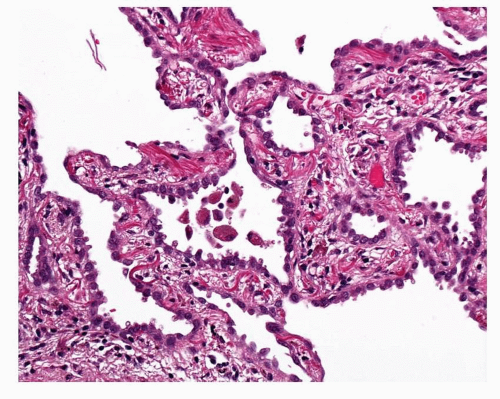 FIGURE 27-7 This example of AAH shows notably thickened alveolar walls with prominent elastic fibers. This is also a relatively cellular lesion with hobnail cells. |
AAH lesions vary in their cellularity. Some lesions may be encountered where there are few or no intercellular gaps, where more of the cells have a columnar or hobnail morphology and pleomorphism is a little more notable (Fig. 27-8). Occasional tiny tufts of cells may be seen but widespread stratification or papillary structures suggest LNMBAC. Such lesions tend to be slightly larger and show more interstitial fibrosis. The WHO classification recommends against separating AAH lesions into low or high grade, largely because there are no agreed, robust criteria for making the distinction. Nonetheless, some authors do make reference to low- and high-grade AAH. It is inevitable that there are “lower” and “higher” grade lesions, as poorly defined as these might be, if we accept that at least a proportion of AAH lesions progress to become what is recognized as LNMBAC, that is adenocarcinoma in situ. This is an arbitrary division of what is a biological continuum of disease. Criteria have been suggested for distinguishing AAH from LNMBAC and
these are discussed in the later section on differential diagnosis of AAH. There will be lesions that just fall short of being classifiable as LNMBAC, yet another “gray area” in this subjective discipline of surgical histopathology.
these are discussed in the later section on differential diagnosis of AAH. There will be lesions that just fall short of being classifiable as LNMBAC, yet another “gray area” in this subjective discipline of surgical histopathology.
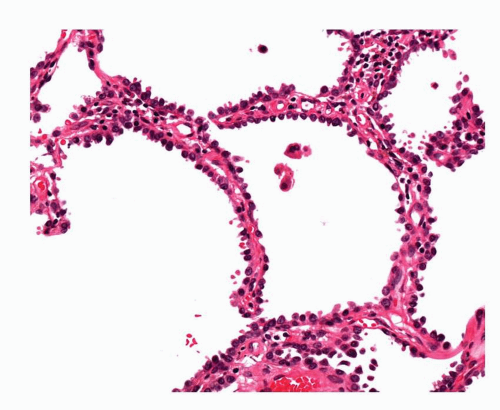 FIGURE 27-8 A more cellular AAH lesion with hobnail cells. This and the lesion in Figure 27-7 are higher grade AAH lesions but still falling short of features of localized nonmucinous bronchioloalveolar carcinoma (adenocarcinoma in situ). |
When AAH was first included in the WHO classification of lung tumors in 1999, it was stated that AAH always measured <5 mm in diameter. This was based upon Roberta Miller’s assertion, as discussed above, that any lesion over 5 mm in diameter should be regarded as LNMBAC.3 Not infrequently lesions are encountered which show the pathological features of AAH described above, which do not fulfill the suggested criteria for a diagnosis of LNMBAC, as discussed below, and which measure over 5 mm. This is the reason for the change in the definition of AAH published in 2004. It is true that AAH usually measures <5 mm across, around 65% to 75% of lesions measure 3 mm or less. Between 10% and 20% of lesions measure over 5 mm. About 10% of AAH lesions the authors measured were over 10 mm in diameter.18 AAH lesions measuring up to 24 mm have been described.19 As mentioned above, larger lesions also tend to be of “higher” grade, although this is not always the case.
The importance of properly prepared material cannot be overemphasized; some of the early skepticism around the existence of AAH stemmed from inadequate sampling of poorly inflated lung tissues. The presence of concurrent inflammation, fibrosis, or emphysema makes lesion identification very difficult. One must have a relatively high threshold for making the diagnosis of AAH; many possible lesions may be dismissed due to uncertainty. As is described below, the vast majority of AAH lesions described by pathologists have been found in the lung resected for carcinoma, especially adenocarcinoma. AAH may be found in any alveolated lung sample, provided there is sufficient expanded tissue present to allow tissue identification. AAH would be a very difficult diagnosis in a small transbronchial lung biopsy sample. It is unlikely that sufficient alveolated lung would be present to demonstrate the lesion and surrounding alveoli. At best, AAH-like areas could be described. AAH cannot be diagnosed by cytology. Various cytological samples may contain populations of atypical bronchioloalveolar cells but given the importance of architecture in the diagnosis of AAH and LNMBAC, a diagnosis of “atypical bronchioloalveolar cell proliferation” is all that should be offered.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree