Chapter 21 Atazanavir
Atazanavir is a relatively new protease inhibitor (PI) that is dosed once daily with or without ritonavir boosting. Since its approval atazanavir has been used in patients naive to antiretroviral therapy and in those patients who are antiretroviral-experienced. Its use in deep salvage therapy is limited. The drug has a favorable lipid profile, even when boosted with low dose ritonavir. This chapter will review the preclinical and clinical experience of atazanavir with emphasis on its efficacy, safety, and resistance profile.
STRUCTURE
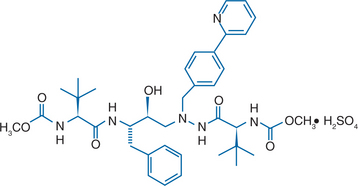
Atazanavir sulfate (Reyataz; BMS-232632) is an inhibitor of HIV-1 protease. It is an azadipeptide analog and bis (L-tert-leucine) derivative.1 The chemical name of atazanavir is (3S,8S,9S,12S)-3,12-bis (1,1-dimethylethyl)-8-hydroxy-4,11-dioxo-9-(phenylmethyl)-6-[[4-(2-pyridinyl)phenyl]methyl]-2,5,6,10,13-pentaazatetradecanedioic acid dimethyl ester, sulfate (1:1) (Fig. 21-1).
IN VITRO ACTIVITY AND MECHANISM OF ACTION
Like other PIs, atazanavir selectively inhibits the virus-specific processing of viral gag and gag-pol polyproteins in HIV-1 infected cells, thus preventing formation of mature virions.2 Atazanavir exhibits potent anti-HIV activity against a variety of laboratory and clinical HIV-1 isolates grown in cell culture, with a 50% effective concentration (EC50; inhibition of 50% of viral replication) in the absence of human sera of 2.6–5.3 nmol/L and an EC90 of 9–15 nmol/L.2 In vitro data suggest that atazanavir exerts more potent anti-HIV-1 protease inhibition than indinavir (EC50 of 5.0–28.7 nmol/L), nelfinavir (6.1–25.9 nmol/L), ritonavir (43.1–129 nmol/L), saquinavir (5.3–24.8 nmol/L), and amprenavir (16.6–54.3 nmol/L).2 In addition, atazanavir is highly selective and has a strong affinity for HIV-1 protease with an inhibition constant (Ki) of 2.7 nmol/L.
Studies on two-drug combinations in peripheral blood mononuclear cells showed that atazanavir had additive antiviral activity with the PIs indinavir, nelfinavir, ritonavir, saquinavir, and amprenavir, as well as the nucleoside reverse transcriptase inhibitors (NRTIs) stavudine, didanosine and lamivudine.2 Atazanavir displayed weak synergistic antiviral activity with the NRTI zidovudine. No enhanced cytotoxic effects were observed at the highest concentrations used for antiviral evaluation.
PHARMACOKINETICS
Atazanavir is rapidly absorbed in the stomach after oral administration of 400 mg qd, with a Tmax of ∼2.5 h in healthy people and 2.0 h in those infected with HIV.3 Steady state is achieved between 4 and 8 days in both healthy volunteers and patients with HIV infection.4 Multiple-dose administration of atazanavir results in ∼2.3-fold accumulation of the drug.
The pharmacokinetics of atazanavir are nonlinear.3–6 Indeed, in a substudy of a phase II trial of patients with HIV receiving HAART, the mean Cmax and area under the curve (AUC) values of atazanavir 200–500 mg increased in a less than dose-proportional manner.5 In contrast, a single-dose study in healthy volunteers demonstrated that there was a greater than dose-proportional increase in atazanavir Cmax and AUC.6
Food has a significant effect on the absorption of atazanavir by enhancing its bioavailability and reducing its pharmacokinetic variability. A single 400 mg dose of atazanavir taken with a light meal (357 kcal; 8.2 g fat, 10.6 g protein) resulted in a 70% increase in AUC and a 57% increase in Cmax relative to the fasting state.1,7 In addition, the inter-individual variability in drug levels was reduced by 40%. Administration of a single 400 mg dose of atazanavir with a high-fat meal (721 kcal; 37.3 g fat, 29.4 g protein) resulted in a mean increase in AUC of 35% with no change in Cmax relative to the fasting state. Thus, atazanavir should be administered with food to reduce pharmacokinetic variability and enhance bioavailability.3
Atazanavir is 86% bound to human serum proteins (alpha-1-acid glycoprotein and albumin).4 Protein-binding is independent of plasma drug concentration. In a multiple dose study in HIV-infected patients dosed with atazanavir 400 mg qd with a light meal for 12 weeks, atazanavir was detected in the cerebrospinal fluid (CSF) and semen.8 As both the semen and CSF atazanavir concentrations were greater than the EC50 values observed for wild-type virus after 12 weeks’ treatment with atazanavir 400 mg, atazanavir should have antiviral activity in these compartments.
Atazanavir is metabolized to two oxygenated, minor metabolites by the hepatic cytochrome P450 liver complex, mainly by the 3A4 isoenzyme (CYP3A4),3,5 which are then excreted in the bile in either free or glucuronidated forms. Additional minor metabolic pathways consist of N-dealkylation and hydrolysis. At clinically relevant concentrations, atazanavir is a moderate competitive inhibitor of CYP3A4 and uridine diphosphate glucuronosyl-transferase 1A1 (UGT 1A1), the enzyme involved in the liver clearance of bilirubin.
Following a single 400 mg dose of 14C-atazanavir, 79% of the total radioactivity was excreted in the feces and 13% in the urine, suggesting that biliary elimination, rather than the kidneys, is a major pathway for the elimination of atazanavir and/or a fraction of the dose is unabsorbed.9 Approximately 20% of atazanavir is excreted unchanged in the feces and 7% in the urine. The mean elimination half-life of atazanavir following a dose of 400 mg qd with a light meal in healthy volunteers and HIV-infected adult patients was ∼7 h at steady state.3 The elimination half-life of atazanavir more than doubled in healthy subjects when administered with once-daily ritonavir 100 mg and a light meal (18.1 vs 7.9 h). The increase was more modest in HIV-infected adult patients (8.6 vs 6.5 h).
A study of the pharmacokinetics of atazanavir in young (18–40 years) and elderly (∼65 years of age) healthy subjects found that there were no clinically important pharmacokinetic differences due to age or gender.3 The pharmacokinetics of atazanavir in children is being investigated. Currently, there is insufficient information to recommend a dose in pediatric patients.
As the kidneys play a minor role in the elimination of atazanavir, the dose of atazanavir does not need to be modified. In adult subjects with moderate to severe hepatic impairment, however, a 45% increase in AUC was observed in hepatically impaired subjects compared with healthy subjects after a single 400 mg dose of atanazavir.3 The mean half-life of atazanavir in hepatically impaired subjects was 12.1 h compared to 6.4 h in healthy volunteers. As increased concentrations of atazanavir are expected in patients with moderate to severe hepatic impairment, a reduction in dose is warranted in subjects with mild to moderate hepatic impairment. Atazanavir is not recommended in patients with severe hepatic impairment. The pharmacokinetics of atazanavir in combination with ritonavir have not been studied in subjects with hepatic impairment.
DRUG INTERACTIONS
Many drug interactions with atazanavir occur via inhibition or induction of the hepatic enzymes responsible for its metabolism, i.e., CYP3A. In addition, atazanavir is an inhibitor of UGT1A1. Co-administration of atazanavir and drugs primarily metabolized by CYP3A (e.g., calcium channel blockers, HMG-CoA reductase inhibitors, immunosuppressants, and PDE5 inhibitors) or UGT1A1 (e.g., irinotecan) may therefore result in increased plasma concentrations of the co-administered drug that could increase or prolong both its therapeutic and adverse effects. Co-administration of atazanavir and drugs that induce CYP3A, such as rifampin, may decrease atazanavir plasma concentrations and reduce its therapeutic effect. Conversely, co-administration of atazanavir and drugs that inhibit CYP3A may increase atazanavir plasma concentrations. A list of drugs that should not be co-administered with atazanavir is shown in Table 21-1.
Table 21-1 Drugs That Should not be Co-Administered With Atazanavir
| Drug Class | Specific Drugs | Reasons |
|---|---|---|
| Contraindicated Drug Combinations | ||
| Benzodiazepines | Midazolam, triazolam | Potential for prolonged/increased sedation, respiratory depression |
| Ergot derivatives | Dihydroergotamine ergotamine, ergonovine, methylergonovine | Acute ergot toxicity (e.g., peripheral vasospasm, ischemia of extremities and other tissues) |
| GI motility agents | Cisapride | Potential for cardiac arrhythmias |
| Neuroleptics | Pimozide | Potential for cardiac arrhythmias |
| Unadvisable Drug Combinations | ||
| Antimycobacterials | Rifampin | Decreases plasma concentrations and AUC of atazanavir |
| Antineoplastics | Irinotecan | Atazanavir may affect the metabolism of irinotecan, increasing its toxicity |
| Herbal products | St Johns wort (Hypericum perforatum) | May reduce plasma concentrations of atazanavir |
| HMG-CoA reductase inhibitors | Lovastatin, simvastatin | Potential for serious reactions such as myopathy |
| Protease inhibitors | Indinavir | Atazanavir and indinavir each associated with indirect (unconjugated) hyperbilirubinemia. No studies conducted on co-administered drugs |
| Proton pump inhibitors | All members | Expected to substantially decrease plasma concentrations of atazanavir |
Data from Bristol-Myers Squibb Company. Reyataz® (atazanavir sulfate) capsules prescribing information. Online. Available: http://www.bms.com 2006.
As the absorption of atazanavir requires an acidic medium, drugs that increase the gastric pH will reduce the solubility of atazanavir and may therefore decrease its plasma concentration.3 Reduced plasma concentrations of atazanavir are thus expected if antacids, buffered medications, H2-receptor antagonists, and proton pump inhibitors are co-administered. Indeed, it is recommended that atazanavir not be administered with any proton pump inhibitor. H2-receptor antagonists, such as ranitidine or famotidine, can be used but only if administered 12 h away from the time of atazanavir administration. In addition, atazanavir should be given 1 h after or 2 h before antacids and buffered medications.
EFFICACY
The therapeutic efficacy of atazanavir in combination with other antiretroviral agents for HIV infection has been investigated in controlled trials in antiretroviral (ART)-naive10–14 or ART-experienced15–18 patients with HIV infection.
First-Line Therapy
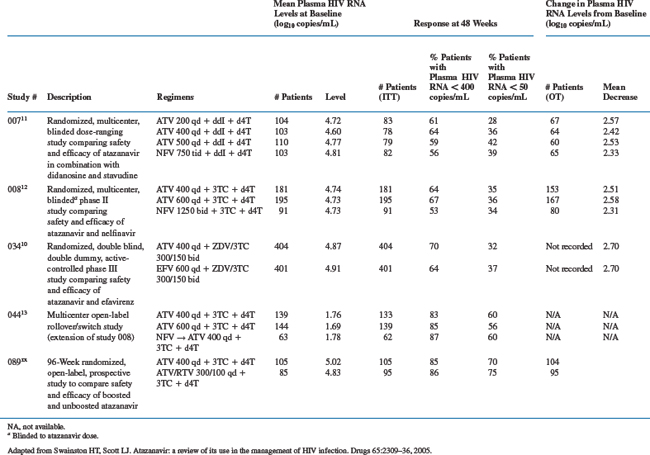
There are three pivotal 48-week studies (007, 008, and 034) that have studied the efficacy and safety of atazanavir regimens in drug-naive patients.10–12 Studies 007 and 008 compared different doses of atazanavir versus nelfinavir along with a dual-NRTI backbone of didanosine (ddI) with stavudine (d4T) or lamivudine (3TC) with stavudine (d4T), respectively.11,12 Study 034 compared atazanavir with efavirenz in combination with zidovudine + 3TC as the nucleoside backbone.10 The phase III study (034) had a 24-week, open-label extension trial for patients who completed at least 48 weeks’ treatment.12,13 Patients continued to receive the same NRTI backbone and once-daily atazanavir 400 or 600 mg, with those previously receiving nelfinavir switched to atazanavir 400.
Study 089 is a 96-week, randomized, open-label prospective study to compare the efficacy and safety of once-daily atazanavir (400 mg) and atazanavir with ritonavir (300 mg/100 mg) both in combination with lamivudine and extended-release stavudine in ART-naive HIV patients. Patients were eligible for inclusion if their plasma HIV RNA levels were 2000 copies/mL; there were no CD4+ T-lymphocytecount restrictions.14
In the pivotal trials, patients were considered ART-naive if they had previously received treatment with an NRTI for 30 days and/or a non-NRTI (NNRTI) or PI for 7 days,10,12 and no ART had been administered within 30 days of screening. Patients were eligible for inclusion in the studies if their plasma HIV RNA levels were 2000 copies/mL and CD4+ T-lymphocyte counts were 100 cells/μL or 75 cells/μL in the absence of a prior diagnosis of AIDS.10,12 Additional inclusion criteria for the extension study included HIV RNA levels <10 000 copies/mL.13
Reductions in HIV viral load of ∼2.5 log10 copies/mL were achieved by week 12 in study 034, week 16 in study 007 and week 24 in study 008.10–12 In all three pivotal studies, the mean reductions from baseline in plasma HIV RNA levels were not significantly different between once-daily atazanavir 400 mg and two or three times-daily nelfinavir or once-daily efavirenz, although responses in both the atazanavir and efavirenz arms in study 034 were artificially low owing to defects in the blood sample tubes (Table 21-2). Viral suppression was maintained during prolonged follow-up.13
The proportion of patients who achieved a reduction in viral load below the limit of quantification (LOQ) at 48 weeks with atazanavir 400 mg was similar to that observed in the nelfinavir and efavirenz groups. Indeed, 64–70% of patients who received atazanavir 400 mg had HIV RNA levels <400 copies/mL compared with 53–64% with the other regimens (Table 21-2). Furthermore, in the 034 study, 32–36% of atazanavir-treated patients had an HIV RNA level <50 copies/mL compared with 34–39% in the other treatment groups. Apparent low levels of response at the <50 copies/mL level are thought to be related to the use of unsuitable sampling tubes at some study sites.
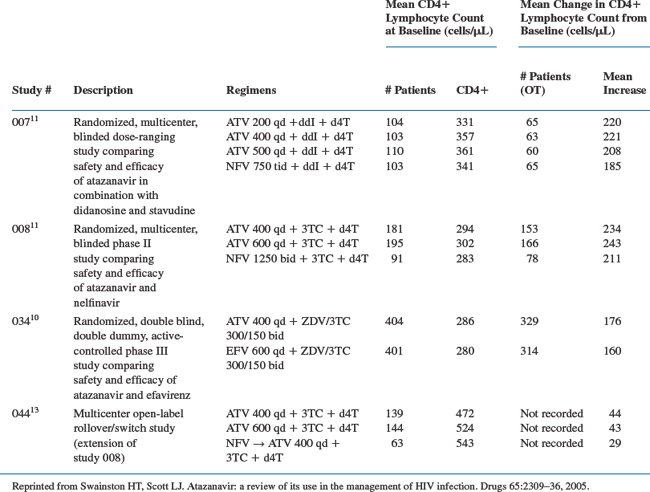
In study 034, the mean increase from baseline in CD4+ T-lymphocyte count in atazanavir-treated patients were numerically greater than that with efavirenz (Table 21-3).10 The effects of ART on CD4+ T-lymphocyte count were similar in patients receiving atazanavir and nelfinavir in studies 007 and 008.11,12
In study 089, the primary endpoint at week 48 was the proportion of patients with HIV RNA <400 log10 copies/mL. This was very similar in both arms of the study (intent-to-treat (ITT) analysis); 85% for atazanavir 400 mg vs 85% for atazanavir/ritonavir 300/100 mg (Table 21-2). Attaining HIV RNA <50 log10 copies/mL at 48 weeks was a secondary endpoint in the study and was achieved by 70% of patients in the atazanavir-only arm and 75% of patients in the boosted atazanavir arm. When evaluating the ITT analysis of this study, itis important to note that elevations in bilirubin were deemed a failure, and more patients went off-study owing to bilirubin elevation in the ritonavir-boosted group versus the unboosted group (8% vs 1 % respectively) thereby having significant impact on the outcomes. The mean increase in CD4+ T-lymphocyte count indicated a strong immunologic response in both arms of the study (+224 cells/mL in the atazanavir only arm versus +189 cells/mL in the boosted atazanavir arm).14
Salvage Therapy
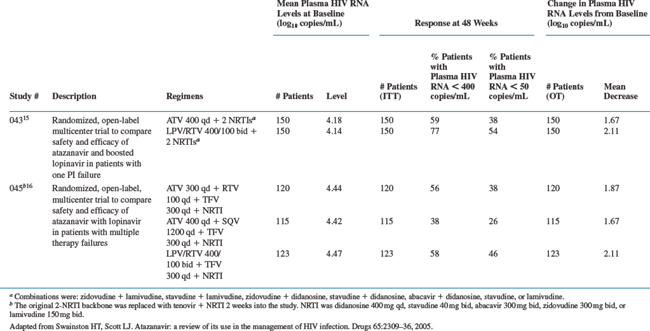
Three randomized, open-label, multicenter, phase III clinical trials (043, 045, and 097) have investigated the beneficial effects and safety of atazanavir for HIV infection in patients with prior virologic failure to PI-containing regimens.15,16,18 In study 043, patients were given once-daily atazanavir 400 mg versus twice-daily lopinavir/ritonavir 400 mg/100 mg for 24 weeks, along with a dual nucleoside backbone selected with the assistance of resistance testing.16 Study 045 was a 48-week trial of adult patients who had failed two or more prior HAART regimens.15 Patients were randomized to one of three groups; once-daily atazanavir 300 mg boosted with once-daily ritonavir 100 mg, once-daily atazanavir 400 mg plus once-daily saquinavir capsules 1200 mg, or twice-daily lopinavir/ritonavir 400/100 mg.15 All treatment groups received once-daily tenofovir 300 mg plus an NRTI. Patients in study 097 received atazanavir, unboosted or boosted with ritonavir, or to their prior PI-based therapy; all groups received a backbone of two NRTIs.18
Patients in studies 043 and 045 had a baseline plasma HIV RNA level of 1000 copies/mL and a CD4+ T-lymphocyte count of 50 cells/μL and were highly ART experienced.15,16 Additional inclusion criteria for study 043 were that patients were currently failing a HAART regimen containing at least one PI, and study 045 required patients to have failed at least two prior HAART regimens that included at least one drug from each ART class (PI, NRTI, and NNRTI) and have serum creatinine, lipase, ALT and AST, and total bilirubin levels of 1.5, 1.4, 3 or 1.5 times less than the upper limit of normal, respectively. In study 043, the median time of prior exposure to any NNRTI was 2.6 years in the atazanavir group and 1.6 years in the lopinavir/ritonavir group, and to any PI or NRTI in was ∼2.6 years and 3.1 years, respectively, for all patients.16
Patients enrolled into study 097 were achieving virologic suppression with a PI-based regimen.18 Indeed, they had had HIV RNA levels <50 copies/mL for at least 3 months and a CD4+ T-lymphocyte count >50 cells/μL.18 Previously, patients had received a PI-containing regimen for a mean duration of 3.4 years, NRTI-based therapy for 3.9 years and an NNRTI-containing regimen for 1.3 years. Additional inclusion criteria for study 097 were that patients had received their first or second HAART regimen, and had no previous virologic failure on a PI-based regimen, and their current regimen included a PI with or without ritonavir, taken at least twice daily or with a pill burden of at least three tablets/day.
After 48 weeks of treatment in study 045, atazanavir boosted with ritonavir was as effective as lopinavir/ritonavir (Tables 21-4 and 21-5).15 Indeed, mean reductions in plasma HIV RNA from baseline for atazanavir/ritonavir and lopinavir/ritonavir were comparable at 1.93 and 1.87 log10 copies/mL, respectively. Virologic suppression and comparable efficacy was maintained through to week 96, when 72% of patients in the boosted atazanavir and lopinavir/ritonavir groups achieved HIV RNA levels <50 copies/mL.17 In addition, the mean change from baseline in HIV RNA levels at week 96 was −2.29 and −2.08 log10 copies/mL in the atazanavir/ritonavir and lopinavir/ritonavir groups, respectively. Efficacy of atazanavir/saquinavir was lower than that of lopinavir/ritonavir in terms of viral load reduction and increases in CD4+ T-lymphocyte count, resulting in premature discontinuation of this arm.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree