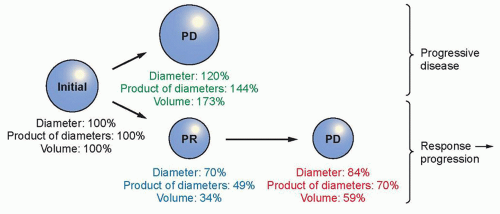
|
WHO3 |
RECIST 1.04 |
RECIST 1.15 |
CNS RANO Criteria7 |
RECIST Mesothelioma8 |
RECIST Immunotherapy9 |
Dimension |
Uni- and bidimensional |
Unidimensional |
Unidimensional |
Bidimensional |
Unidimensional |
Bidimensional |
Measurable Lesion |
Not defined |
Longest diameter, ≥20 mm with most modalities; ≥10 mm with spiral CT |
Longest diameter ≥10 mm on CT or on skin if using calipers; ≥20 mm if using CXR |
Two perpendicular diameters of contrast enhancing lesions ≥10 mm |
Tumor thickness perpendicular to chest wall or mediastinum, measured in two positions at three levels on transverse cuts of CT scan |
Longest perpendicular diameters |
Measurable Lymph Nodes |
Not defined |
Not defined |
≥15 mm short axis |
— |
— |
— |
Disease Burden to be Assessed at Baseline |
All (not specified) |
Measurable target lesions up to 10 total (5 per organ); other lesions nontarget |
Measurable target lesions up to 5 total (2 per organ); other lesions nontarget |
Two to five lesions in patients with several lesions |
Pleural disease in perpendicular diameter; nodal, subcutaneous, and other bidimensional lesions measured unidimensionally as per the RECIST criteria |
5 lesions per organ, up to 10 visceral lesions and five cutaneous lesions |
Sum |
Sum of the products of bidimensional diameters or sum of linear unidimensional diameters |
Sum of longest diameters of all measurable lesions |
Sum of the longest diameters of target lesions with only exception use of short axis for lymph nodes |
Sum of the products of perpendicular diameters of all measurable enhancing target lesions |
Sum of the six measurements defines a pleural unidimensional measure |
SPD with new lesions incorporated into baseline; tumor burden = SPDindex lesions + SPDnew lesions |
Complete Response |
Disappearance all known disease |
Disappearance all known disease |
Disappearance all known disease; lymph nodes <10 mm |
— |
Disappearance all target lesions with no evidence of tumor elsewhere |
Disappearance all lesions in two consecutive observations |
Partial Response |
≥50% decrease |
≥30% decrease; all other no evidence of progression |
≥30% decrease; all other disease, no evidence of progression |
≥50% reduction; stable or decreased steroid use compared to baseline |
≥30% reduction in total tumor measurement |
≥50% decrease compared with baseline in two observations |
Response Confirmation? |
≥4 weeks apart |
≥4 weeks apart |
≥4 weeks apart (if response primary end point); no, if secondary endpoint |
≥4 weeks apart |
Repeat on two occasions ≥4 weeks apart |
≥4 weeks apart |
Progressive Disease |
≥25% increase in size of one or more measurable lesions or appearance of new lesions |
≥20% increase, taking as reference smallest sum in study; or appearance of new lesions |
≥20% increase, with absolute increase ≥5 mm, taking as reference smallest sum in study; or appearance of new lesions |
≥25%, or any new lesions |
≥20% increase in the total tumor measurement over the nadir measurement, or the appearance of one or more new lesions |
≥25% increase compared with nadir confirmed ≥4 weeks apart; up to five new lesions (≥5 × 5 mm) per organ incorporated into tumor burden |
|
Nonmeasurable disease: Estimated increase of ≥25% |
Nonmeasurable disease: unequivocal progression |
Nonmeasurable disease: unequivocal progression |
Nonmeasurable disease: >5 mm increase in maximal diameter; ≥25% increase in SPD; or significant increase in nonenhancing lesions on same or lower dose of corticosteroids |
— |
New, nonmeasurable lesions (i.e., <5 × 5 mm) do not define progression |
Stable Disease |
Stable disease or non-PR and non-PD ≥4 weeks |
Non-PR, non-PD; minimum time defined by protocol |
Non-PR, non-PD; minimum time defined by protocol |
— |
Non-PR, non-PD |
Non-irPR, non-irPD |
CXR, Chest X-ray; SPD, sum of products of two largest perpendicular diameters; PD, progressive disease; irPR, immune-related partial response; irPD, immune-related progressive disease. |