Figure 173.1 Photomicrograph demonstrating Aspergillus hyphae in a lung at autopsy in a liver transplant patient who died of systemic aspergillosis. The hyphae are stained with methenamine silver. (Courtesy of Dr. Barbara Banner, University of Massachusetts Medical School.)
Clinical manifestations and diagnosis of invasive aspergillosis
Although inhalation of conidia is common, invasive disease is relatively rare. The vast majority of affected patients are severely immunosuppressed. Major risk factors include prolonged neutropenia and macrophage dysfunction secondary to cytotoxic chemotherapy and high doses of corticosteroids, respectively. In patients who have undergone hematopoietic stem cell transplantation, additional risk factors are graft-versus-host disease and cytomegalovirus infection. Invasive aspergillosis also is particularly common in individuals with chronic granulomatous disease, a rare genetic disorder characterized by a defective phagocyte respiratory burst. Finally, critically ill patients appear to be at risk for invasive aspergillosis, even without aforementioned risk factors.
Invasive pulmonary aspergillosis, with or without dissemination, is the most common form of disease. Signs and symptoms of invasive aspergillosis are nonspecific. Fever is generally present. Radiographic features include patchy densities or well-defined nodules that may be single or multifocal and can progress to cavitation or consolidation. High-resolution computed tomography (CT) scanning has greater sensitivity than plain films. Macronodules (nodules greater than 1 cm in diameter) are present in the vast majority of patients with invasive pulmonary aspergillosis. Nodules may be surrounded by a perimeter of ground-glass opacity, the so-called halo sign (Figure 173.2). Cavitation (“air-crescent sign”) is less common and tends to occur as a later manifestation of disease. Tracheobronchitis without alveolar invasion may be seen, particularly in lung transplant recipients and those with advanced acquired immunodeficiency syndrome (AIDS). Invasive Aspergillus sinusitis is the second most common manifestation and must be distinguished from saprophytic colonization. Its clinical features include fever, localized pain, proptosis, and visual problems. Fungal keratitis due to direct inoculation of Aspergillus has been increasingly recognized as an important cause of visual loss, particularly among agrarian workers in developing countries. Less common manifestations include cutaneous aspergillosis, which may be seen at intravenous catheter insertion sites in neutropenic patients or at sites of burn wounds. While disseminated aspergillosis can involve any organ, cerebral involvement is a particularly common manifestation. Endocarditis due to Aspergillus is relatively rare and can be difficult to diagnose as blood cultures are often negative.
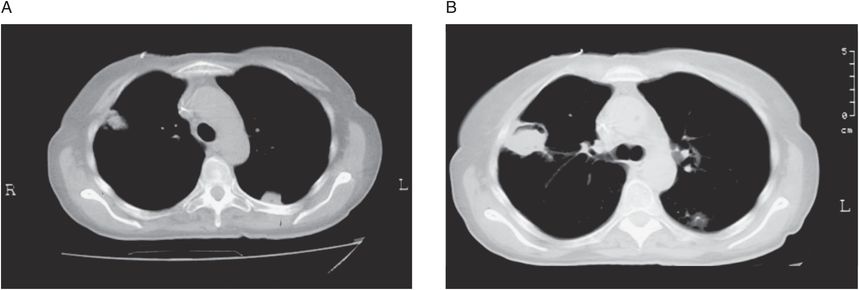
Figure 173.2 High-resolution computed tomography scans from patients with invasive pulmonary aspergillosis. A: Two pleural-based nodules can be seen. The one on the right is surrounded by a gray area of low-attenuation (halo sign). B: Air-crescent sign in a patient recovering from neutropenia. (Courtesy of the Aspergillus/Aspergillosis website: www.aspergillus.org.uk. Copyright by the Fungal Research Trust. Used with permission.)
Invasive aspergillosis must be strongly suspected in any high-risk patient with fever unresponsive to broad-spectrum antibiotics, and empiric antifungal therapy should be considered. Although Aspergillus can be a laboratory contaminant or a colonizer, a positive culture for Aspergillus in an at-risk patient is predictive of invasive disease and should not be ignored. However, in patients with established disease, cultures and even biopsies are often negative.
In an effort to improve prognosis with earlier detection, nonculture methods to detect Aspergillus antigens and nucleic acids in specimens from high-risk patients have been studied. Tests to detect two cell wall antigens released by growing aspergilli, galactomannan and β-glucan, are clinically available. Serum galactomannan levels have excellent specificity but only moderate sensitivity. Sensitivity is higher in patients with hematologic malignancies, where positive results may precede clinical or radiologic manifestations, than in those with solid organ transplants. False positives have been noted in patients receiving piperacillin–tazobactam and amoxicillin–clavulanate due to contamination of some lots with galactomannan. False negatives are considerably more common in patients receiving anti-mold prophylaxis. Assays to detect β-glucans in clinical specimens appear to perform comparably to those for galactomannan, with the caveat that elevated β-glucans may be seen in fungal infections besides aspergillosis. Tests to detect Aspergillus DNA and RNA in clinical samples remain investigational. It should be emphasized that the aforementioned surrogate detection markers cannot definitively establish or exclude a diagnosis of aspergillosis and clinical correlation is required. Moreover, testing for antibodies, while useful for other forms of aspergillosis (see below), is not helpful in invasive aspergillosis.
Treatment of invasive aspergillosis
Licensed drugs with activity against Aspergillus include amphotericin B (a polyene); the triazoles, itraconazole, voriconazole, and posaconazole; and the echinocandins, caspofungin, micafungin, and anidulafungin. Fluconazole and ketoconazole do not inhibit Aspergillus at clinically obtainable concentrations and should not be used to treat aspergillosis. It is somewhat difficult to rank the active drugs in terms of relative efficacy because few randomized comparative trials have been performed. Studies based on comparisons to historical controls are problematic due to significant differences in the patient populations. A multicenter, randomized trial compared voriconazole with amphotericin B in 277 patients with definite or probable acute invasive aspergillosis. The voriconazole regimen demonstrated superior efficacy and a 22% relative survival benefit. Moreover, there were fewer treatment-related adverse events in the voriconazole group. This study was criticized for its unblinded design, the use of conventional (rather than lipid-based) amphotericin B preparations, and the allowance of a switch to other licensed antifungal medications. Nevertheless, the large survival benefit observed established voriconazole as the drug of choice for initial treatment of invasive aspergillosis. An important caveat though is voriconazole is not effective therapy against zygomycosis, which can present in a similar manner to aspergillosis.
In the above study, two doses of 6 mg/kg of intravenous voriconazole were administered on day 1, followed by 4 mg/kg/day. A switch to oral voriconazole at a dose of 200 mg twice a day was allowed after 1 week. Transient visual disturbances, including blurred vision, altered color perception, and photophobia, are common with voriconazole and tend to resolve without incident. Other side effects that have been observed include rash and liver function test abnormalities. Voriconazole is a substrate and an inhibitor of CYP2C19, CYP2C9, and CYP3A4. Thus, drug interactions are common and dose adjustments of voriconazole and coadministered drugs may be needed. The authors recommend obtaining steady-state trough levels and adjusting the dosing if necessary to achieve levels between 1.0 and 5.5 μg/mL. In patients with an estimated creatinine clearance <50 mL/min, accumulation of the intravenous vehicle occurs; these patients should be given oral voriconazole whenever possible.
In addition to voriconazole, other licensed triazoles with activity against Aspergillus are itraconazole and posaconazole. Itraconazole appears to be least potent and is mainly used for the treatment of aspergillomas and allergic bronchopulmonary aspergillosis (see below). Posaconazole appears more promising and has the added advantage of having useful activity against zygomycetes, but clinical experience with its use for primary treatment of invasive aspergillosis is limited. Delayed-release tablets and an IV formulation of the drug recently became available. Resistance of Aspergillus to voriconazole and other triazole drugs can emerge during therapy although the extent to which this leads to clinical failure is not clear. Ominously, primary resistance has been increasingly reported in both environmental and initial clinical isolates of Aspergillus, perhaps as a consequence of the agricultural use of azoles.
Amphotericin
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree





