Fig. 10.1
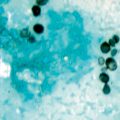
Microscopic morphology of Aspergillus fumigatus showing a single role of phialides (uniseriate) bearing smooth conidia in a columnar fashion. (Courtesy of www.doctorfungus.org)
Table 10.1
Characteristics of common Aspergillus species
Aspergillus species | Mycological characteristics | Clinical significance | Mycoses |
|---|---|---|---|
A. flavus | Olive to lime green colonies | Second most common species, produces aflatoxin, may be less susceptible to polyenes | Sinusitis, cutaneous infection, pulmonary, and disseminated disease |
A. fumigatus | Smoky, blue- or gray-green, small, smooth conidia (2–2.5 µm) | Most common species causing invasive infection | Invasive pulmonary aspergillosis, disseminated infection, CNS, others |
A. niger | Typically black colonies, radiate conidial head, large rough conidia | Common cause of otomycosis, produces oxalate crystals which may be seen in host | Otomycosis, cutaneous, endophthalmitis, aspergilloma, invasive pulmonary, or disseminated disease less common |
A. terreus | Beige to buff colonies, globose accessory conidia along hyphae | Increasing frequency, associated with soil, usually resistant to polyenes | Pulmonary, disseminated, cutaneous, keratitis, CNS |
A. lentulus | Poorly sporulating variant of A. fumigatus | May be multidrug resistant, recently described variant, may be underdiagnosed | Invasive pulmonary, disseminated, other sites |
Epidemiology
The incidence of invasive aspergillosis has increased substantially during the past few decades because of the use of more intensive cytotoxic anticancer chemotherapy and the introduction of novel immunosuppressive therapies for organ transplant recipients, both of which have prolonged the period of risk for many individuals. The increasing number of patients undergoing solid organ, bone marrow, and hematopoietic stem cell transplantation, and the implementation of aggressive surgical interventions has also contributed to the increased incidence [9]. The changes in epidemiology of invasive aspergillosis may also be the result of growing awareness of aspergillosis among clinicians, the introduction of noninvasive diagnostic tools and improved microbiological laboratory techniques.
Invasive fungal infections are an important cause of morbidity and mortality among patients with severely compromised immune systems. Although there have been significant advances in the management of immunosuppressed patients, invasive aspergillosis remains an important life-threatening complication, and is the leading cause of infection-related mortality in many immunocompromised individuals [13].
Immunosuppression and breakdown of anatomical barriers, such as the skin, are the major risk factors for fungal infections [7]. Individuals at risk for invasive aspergillosis include those with severely comprised immune systems as a result of anticancer chemotherapy, solid organ or bone marrow transplantation, AIDS, or use of high-dose corticosteroids. Patients with hematological disorders, such as prolonged and severe neutropenia, those undergoing transplantations, and those treated with corticosteroids and newer immunosuppressive therapies such as the tumor necrosis factor-α antagonists (e.g., inflixamab) are considered to be at highest risk for invasive aspergillosis [7, 14].
Pathogenesis and Immunity
Invasive aspergillosis most frequently originates via inhalation of Aspergillus conidia into the lungs, although other routes of exposure, such as inhalation of water aerosols contaminated with Aspergillus conidia have been suggested [11].
In the absence of effective pulmonary host defenses, the inhaled small resting conidia enlarge and germinate, then transform into hyphae with subsequent vascular invasion and eventual disseminated infection. The incubation period for conidial germination in pulmonary tissue is variable, ranging from 2 days to months [15]. Hydrocortisone significantly increases the growth rates of Aspergillus; likely one of the reasons corticosteroids pose a risk factor for invasive disease [16].
Although infection in apparently normal hosts can occur, invasive aspergillosis is extremely uncommon in immunocompetent hosts [5]. Normal pulmonary defense mechanisms usually contain the organism in a host with intact pulmonary defenses. The first line of defense against Aspergillus is ciliary clearance of the organism from the airways and limited access to the alveoli due to conidia size. This feature is one reason for the increased pathogenicity of A. fumigatus as compared with other species of Aspergillus [16]. Once conidia reach the alveoli, pulmonary macrophages are generally capable of ingesting and killing Aspergillus conidia [17]. When macrophages fail to kill the conidia (e.g., high-fungal inoculum, decreased number or function of macrophages), conidia germinate and begin to form hyphae. Polymorphonuclear leukocytes are recruited via complement activation and production of neutrophil chemotactic factors and extracellularly kill both swollen conidia and hyphae [18]. Antibodies against Aspergillus are common due to the ubiquitous nature of the organism, although they are not protective nor are they useful in the diagnosis of infection in high-risk patients due to the lack of consistent seroconversion following exposure or infection [19].
Corticosteroids play a major role in increasing susceptibility to Aspergillus by decreasing oxidative killing of the organism by pulmonary macrophages and by increasing the linear growth rate by as much as 30–40 % and cell synthesis by more than 150 % [16].
Many Aspergillus species produce toxins including aflatoxins, ochratoxin A, fumagillin, and gliotoxin. Gliotoxin works in several ways to help evade host defenses:
In tissues, invasive aspergillosis causes extensive destruction across tissue planes via vascular invasion with resulting infarction and necrosis of distal tissues.
Clinical Manifestations
The clinical syndromes associated with aspergillosis are diverse, ranging from allergic responses to the organism including allergic bronchopulmonary aspergillosis (ABPA), asymptomatic colonization, superficial infection, and acute or subacute, and chronic invasive disease. The clinical presentation generally corresponds to the underlying immune defects and risk factors associated with each patient group, with greater immune suppression correlating with the increased risk for invasive disease. Although this chapter focuses on invasive aspergillosis, a brief description of other presentations follows. The reader is encouraged to refer to other sources for more in-depth discussion of those conditions [1].
Allergic Bronchopulmonary Aspergillosis
ABPA is a chronic allergic response to Aspergillus characterized by transient pulmonary infiltrates due to atelectasis. The incidence of ABPA is estimated to range from 1 to 2 % in patients with persistent asthma and approximately 7 % (with a range from 2 to 15 %) of patients with cystic fibrosis [22]. Specific criteria are used to establish the diagnosis of ABPA as no single finding is diagnostic for the condition, although some presentations, like central bronchiectasis in patients with asthma highly suggest the diagnosis [22–24]. ABPA typically progresses through a series of remissions and exacerbations but can eventually lead to pulmonary fibrosis, which is associated with a poor long-term prognosis [24]. Management of ABPA is directed at reducing acute asthmatic symptoms and avoiding end-stage fibrosis. Corticosteroid therapy is commonly used for treating exacerbations although few randomized trials have been conducted for their use [25]. The role for antifungal therapy was evaluated with a randomized, double-blind, placebo-controlled trial that showed itraconazole at 200 mg per day for 16 weeks significantly reduced daily corticosteroid use, reduced levels of immunoglobulin E (IgE), and improved exercise tolerance and pulmonary function [23, 26].
Aspergilloma
A pulmonary fungus ball, due to Aspergillus or “aspergilloma,” is a solid mass of hyphae growing in a previously existing pulmonary cavity, typically in patients with chronic lung disease, such as bullous emphysema, sarcoidosis, tuberculosis, histoplasmosis, congenital cyst, bacterial lung abscess, or, very rarely, in a pulmonary bleb from Pneumocystis pneumonia in AIDS [27, 28]. On chest radiograph, a pulmonary aspergilloma appears as a solid round mass in a cavity. In many patients, the fungus ball due to Aspergillus remains asymptomatic, but in a significant number, hemoptysis occurs and can be fatal [29]. Surgical resection is considered as the definitive therapy, but the dense pleural adhesions adjacent to the fungus ball and the poor pulmonary reserve of most patients with this condition, makes surgery hazardous. Contamination of the pleural space with Aspergillus and the common complication of bronchopleural fistula in the postoperative period can lead to chronic Aspergillus empyema. Dense adhesions make pleural drainage difficult, often requiring pleural stripping or an Eloesser procedure, further compromising lung function [29].
Aspergillus can also be associated with fungal balls of the sinuses without tissue invasion [28]. The maxillary sinus is the most common site for a sinus aspergilloma to occur [28]. Clinical presentation is similar to that for any chronic sinusitis. Management is usually directed at surgical removal and a generous maxillary antrostomy for sinus drainage, along with confirmation that invasive disease has not occurred.
Other Superficial or Colonizing Syndromes
Other superficial or colonizing syndromes of aspergillosis include otomycosis, a condition of superficial colonization typically due to A. niger [30]; onychomycosis which, although rare, can become chronic and respond poorly to antifungal agents [31]; and keratitis, particularly following trauma or corneal surgery [32].
Chronic Pulmonary Aspergillosis
Denning et al. have described three distinct syndromes of chronic pulmonary aspergillosis in order to better characterize those patients who develop chronic pulmonary disease related to Aspergillus [33]. These conditions include chronic cavitary pulmonary aspergillosis, which is characterized by the formation and expansion of multiple cavities, which may contain fungus balls; chronic fibrosing aspergillosis, which as its name suggests involves extensive fibrosis; and chronic necrotizing aspergillosis or subacute aspergillosis, in which slowly progressive infection occurs usually in a single thin-walled cavity. In all of these conditions, the diagnosis is suggested by radiological and clinical features and the role for therapy remains speculative, although it appears that long-term antifungal therapy may be beneficial in a subset of patients, perhaps even with the extended spectrum triazole antifungals [33, 34].
Invasive Pulmonary Aspergillosis
Invasive pulmonary aspergillosis is the most common form of invasive aspergillosis in immunocompromised patients. This infection occurs following approximately 2 weeks of neutropenia [35] or during the course of graft versus host disease, now the most common risk factor in hematopoietic stem cell transplant recipients [36]. Symptoms include fever (may be absent in the presence of high-dose corticosteroid therapy), dry cough, shortness of breath, pleuritic chest pain, hemoptysis, as well as pulmonary infiltrates all of which lag behind disease progression. In lung transplant patients and those with AIDS, Aspergillus tracheobronchitis can present with cough, wheezing, and shortness of breath and chest radiographs show normal lungs with or without atelectasis [37].
Disseminated Aspergillosis
A variety of signs and symptoms are seen with disseminated invasive aspergillosis, based on the organs involved. The organs involved include kidneys, liver, spleen, and central nervous system (CNS; signs and symptoms of stroke or meningitis) most frequently, followed by the heart, bone, skin, and other organs [8]. Aspergillosis of the skin can occur either as a manifestation of disseminated disease or by direct extension from a local inoculation, for example, from an intravenous catheter [38].
Sinusitis
Aspergillosis of the sinuses presents clinically like rhinocerebral mucormycosis, but is more common in neutropenic patients than in those with diabetic ketoacidosis, and inflammatory signs may thus be less frequent. Fever, nasal congestion, facial pain can progress to visual changes, proptosis, and chemosis if the infection spreads to the orbit. Posterior extension to the brain can lead to cranial nerve palsies, other focal neurologic deficits, as well as a depressed level of consciousness [39].
Endocarditis
Aspergillus endocarditis is the second most common form of fungal endocarditis after that caused by Candida species and occurs in prosthetic valve recipients and in native cardiac valves in intravenous drug users and patients with indwelling central venous catheters [40]. Clinically, these patients present with fever and embolic complications. Blood cultures are rarely positive even with extensive disease [41].
Diagnosis
Current diagnostic modalities are limited and the clinician must rely on the combination of knowledge of risk factors, a high index of suspicion, clinical judgment, and the finding of fungi in tissue specimens and/or cultures from the presumed site of infection (Table 10.2). The diagnosis of proven invasive aspergillosis requires both tissue biopsy demonstrating invasion with hyphae and a culture positive for Aspergillus species [42]. Aspergillus produce hyaline, 3–6 µm wide septate hyphae that typically branch at acute angles [43] (Fig. 10.2). In tissue, these features can often distinguish Aspergillus from agents of mucormycosis, but they cannot distinguish Aspergillus from a large number of other opportunistic molds, including Fusarium and Scedosporium (Pseudallescheria). Thus, culture is needed to confirm the diagnosis [43]. Unfortunately, invasive, or even less invasive procedures like bronchoscopy, are often contraindicated in immunosuppressed patients, many of whom have low platelets due to chemotherapy and other complications. In this setting, positive culture can support the diagnosis of invasive aspergillosis.
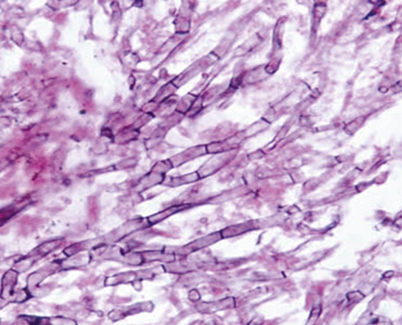

Fig. 10.2
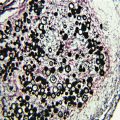
Periodic acid–Schiff (PAS) stained tissue section of lung showing dichotomously branched, septate hyphae of Aspergillus fumigatus. (Courtesy of www.doctorfungus.org)
Table 10.2
Diagnosis of invasive aspergillosis
Diagnostic method | Comment |
|---|---|
Respiratory culture | Not frequently positive early in course of infection; positive result in high-risk patient (bone marrow transplant, neutropenia) highly correlates with infection; may indicate colonization in other populations (chronic pulmonary diseases, lung transplant) |
Galactomannan | Aspergillus Platelia system (BioRad, Redmond, WA) with variable sensitivity—low (~ 40 %) with single samples or prior antifungal therapy, or prophylaxis; better yield with reduced threshold for positivity (> 0.5), serial samples, testing on BAL samples. False positives historically with pipercillin–tazobactam, certain foods, neonates |
1,3-β-D-glucan | Nonspecific detection of cell wall glucan. Commercially available Fungitell™ assay (Associates of Cape Cod, Falmouth, MA), limited validation and availability |
PCR | Remains investigational due to lack of standardized reagents and methods, both false positives and negatives may occur, some recent studies have suggested less sensitive than other assays |
Computed tomography | In high-risk patient, “halo” sign and/or pulmonary nodules without other documented cause may be a frequent and early sign of invasive pulmonary aspergillosis |
Plain chest radiography is of limited utility in invasive aspergillosis as it has low sensitivity and specificity in this disease [6]. In contrast, chest CT scans have proven useful in early diagnosis of invasive pulmonary aspergillosis as the “halo sign” of low attenuation surrounding a pulmonary nodule, has successfully been used as a marker for early initiation of therapy in high-risk patients with neutropenia or who have undergone HSCT [44–46]. Of note, these radiographic findings are also consistent with other infections such as Nocardia species, and may increase over the first week of therapy even when the patient in improving; follow-up scans should be ordered and interpreted cautiously with full attention to the clinical progress of the patient [44].
Nonculture diagnostic tests have also been used to diagnose aspergillosis and in attempts to preempt difficult-to-treat proven disease. A sandwich enzyme immunoassay (EIA) that utilizes a monoclonal antibody to Aspergillus galactomannan (Platelia Aspergillus, BioRad, Redmond, WA) is approved for serum and bronchoalveolar lavage (BAL) fluid and is being used with varying success around the world [47–49]. Questions remain regarding the value of routine surveillance testing, frequency of testing, role of false-positive results (seen in solid organ transplant recipients, patients treated with piperacillin–tazobactam and other medications, and neonates), importance of prior antifungal therapy, and correlation of serum galactomannan results with clinical outcome [50].
Several reports demonstrate the potential for using polymerase chain reaction (PCR) as an early diagnostic marker, which appears more sensitive than other methods including galactomannan [51, 52]. These assays may be associated with false-positive results due to the ubiquitous nature of Aspergillus conidia, are not standardized, and remain investigational at the present time [53–56].
Other nonculture-based methods for the diagnosis of invasive aspergillosis include detection of the nonspecific fungal marker 1,3-β-D-glucan using a variation of the Limulus amebocyte assay. This assay (Fungitell™, Associates of Cape Cod, Falmouth, MA) has been approved for diagnostic purposes by the Food and Drug Administration (FDA) and is a colorimetric assay that can indirectly determine the concentration of 1–3, β-D-glucan in serum samples [57]. The test appears promising as an indicator of infection due to many fungi, including Aspergillus and Candida but not Cryptococcus or Mucorales (which contain little or no β-D-glucan). One study suggested the utility of the assay in early diagnosis of invasive fungal infection in a leukemic population, but validation remains limited [58].
Interpretation of results is complicated with frequent false-positive β-D-glucan results, as well as reports of “interfering substances,” hemodialysis with cellulose membranes, intravenous immunoglobulin, albumin, gauze packing of serosal surfaces, intravenous amoxicillin–clavulanic acid (not available in the USA) [59], and bloodstream infections with certain bacteria, such as Pseudomonas aeruginosa [60, 61]
Treatment
The goals of treatment of patients with invasive aspergillosis are to control infection and to reverse any correctable immunosuppression. Patients at high risk of developing invasive aspergillosis should be treated based on clinical or radiological criteria alone if microbiological or histological diagnosis would significantly delay treatment [2].
Treatment of Aspergillus infection is challenging due to difficulty in diagnosis, the presence of advanced disease in many by the time of diagnosis, and the presence of severe, often irreversible, immunosuppression. Mortality rates are high in patients with invasive aspergillosis and the efficacy of currently available treatments is limited by spectrum of activity, extensive drug–drug interactions, and serious toxicity. Treatment failure with currently available antifungal medication in patients with invasive aspergillosis has been reported to be 40 % or higher in some series [3, 4]. Antifungal therapies with activity against Aspergillus include broad-spectrum triazoles (voriconazole, posaconazole, and isavuconazole), lipid formulations of amphotericin B, and the echinocandins (caspofungin, micafungin and anidulafungin), all of which offer options for therapy of this disease [62, 63] (Table 10.3). Guidelines developed by the Infectious Diseases Society of America and the American Thoracic Society provide summaries of existing data as well as recommendations. Of note, relatively few randomized trials of therapy for invasive aspergillosis have been completed so many recommendations stem from nonrandomized and noncomparative studies, as well as expert consensus [2].
Table 10.3
Antifungal agents for treating invasive aspergillosis
Agent | Typical dose/route of administration | Comments |
|---|---|---|
Azole | ||
Voriconazole | 6 mg/kg IV q12 h x 2 doses, then 4 mg/kg IV q12 h; 200 mg PO bid (weight-based dosing should be considered) | Better efficacy and improved survival compared with amphotericin B deoxycholate; current recommended primary therapy for invasive aspergillosis; drug interactions common, hepatic toxicity (10–15 %) may be dose limiting; visual effects common (~ 30 %) but not usually dose limited and no long-term toxicity reported [98] |
Itraconazole | 200 mg tid for 3 days, then 200 mg PO bid (oral solution) | Second-line agent for invasive aspergillosis; erratic bioavailability, improved with oral solution; drug interactions including chemotherapeutic agents; intravenous formulation no longer available [2] |
Posaconazole | Oral solution—200 mg PO qid loading, 400 PO bid maintenance; extended release tablets—300 mg bid x 2 doses, then 300 mg daily; intravenous—300 mg bid x 2 doses, then 300 mg daily | |
Isavuconazole | Investigational | Full clinical development underway |
Ravuconazole | Investigational | In vitro activity, but limited clinical development at present [63] |
Polyene | ||
Amphotericin B deoxycholate | 1.0–1.5 mg/kg IV daily | Prior “gold standard”; associated with significant toxicity and limited efficacy in severely immunosuppressed patients [100] |
Liposomal amphotericin B | 3–6 mg/kg IV daily | Alternative primary therapy; well tolerated; limited nephrotoxicity or infusion-related reactions; anecdotal reports of efficacy with higher doses (7.5 mg/kg/d or more) |
Amphotericin B lipid complex | 5 mg/kg IV daily | Indicated for salvage therapy or intolerance to standard agents, generally well tolerated [101] |
Amphotericin B colloidal dispersion | 3–6 mg/kg IV daily | Less nephrotoxic than amphotericin B deoxycholate, but associated with more infusion-related and pulmonary toxicity than other lipid formulations [81] |
Echinocandin | ||
Caspofungin | 70 mg x 1 dose, then 50 mg IV daily | Indicated for salvage therapy of aspergillosis, experimental and clinical data for use in combination therapy; well tolerated [84] |
Micafungin | Investigational for aspergillosis (IV) | Used in doses of 100 mg/d in salvage studies; 50 mg/d for prophylaxis; well tolerated [102] |
Anidulafungin | Investigational for aspergillosis (IV) | In vitro activity; studied at doses of 100 mg/d after 200 mg loading dose in other fungi; well tolerated [103] |
Azoles
Voriconazole is a potent, broad-spectrum triazole that has fungicidal activity against many Aspergillus species, including A. terreus, is approved for therapy of invasive aspergillosis, and has replaced amphotericin as the recommended primary therapy for patients with invasive aspergillosis [2, 62, 64]. This recommendation is based on data from a randomized trial that compared voriconazole to conventional amphotericin B for the primary treatment of invasive aspergillosis, with each agent followed by other licensed antifungal therapy if needed for intolerance or progression of disease, in severely immunocompromised patients with invasive aspergillosis [45]. In this trial, voriconazole was superior to amphotericin B with successful outcomes in 52 % of patients as compared to only 31 % in those receiving amphotericin B. In addition, voriconazole demonstrated a survival advantage to amphotericin B with an absolute 13 % difference in mortality between treatment groups.
In clinical trials, voriconazole has been adequately tolerated and the drug exhibits a favorable pharmacokinetic profile. There are a number of issues to consider, including important drug interactions, especially those with immunosuppressive agents, such as cyclosporine, tacrolimus, and sirolimus, the latter of which is contraindicated for use with voriconazole, and intolerance to the drug. The most common adverse event has been a transient and reversible visual disturbance described as an altered perception of light which has been reported in approximately 30 % of the treated patients, but has not been associated with pathological changes [45]. Other adverse events include liver function test abnormalities in 10–15 %, and skin rash in 6 % (sometimes associated with sun exposure). Long-term voriconazole therapy has been associated with skin cancer and periostitis related to high fluoride levels [65–67].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree