and Victor Bernet1
(1)
Mayo Clinic, Jacksonville, FL, USA
Keywords
Thyroid nodulesUltrasoundCarotid DopplerComputed tomography (CT)Thyroid incidentalomasUltrasound examinationNonpalpable nodulesSolitary nodulesObjectives
To review steps in evaluation of a newly discovered thyroid nodule, ultrasound characteristics and indications for fine needle aspiration, to understand cytology results and role of molecular analysis and to discuss management options and long-term follow-up based on nodule characteristics and cytology.
Case Presentation
A 71-year-old Caucasian male is referred to the endocrine department for a newly diagnosed right thyroid nodule found at palpation during a routine physical examination. He had no associated symptoms such as voice changes, hoarseness, and neck swelling. No systemic type symptoms were reported. The patient was without any history of radiation exposure or family history of thyroid cancer. At physical examination, thyroid was normal in size, with irregular surface and a 2 cm discrete nodule palpable in the right lower lobe. No cervical lymphadenopathy was noted. The remaining of the physical examination was unremarkable.
TSH was 1.6 μIU/mL [0.3–5.0 μIU/mL]. An ultrasound of the neck revealed two heterogeneous solid nodules in the mid and lower pole of the right lobe. The largest, located in the lower pole measured 3.0 × 2.1 × 2.3 cm and had coarse internal calcifications, the second, located in the mid pole measured 1.3 × 1.1 × 1.1 cm and had peripheral calcifications. A third nodule, hypoechoic and less than 1 cm in size, was located in the right upper pole. Left lobe contained a single solid nodule measuring 1.2 × 0.9 × 0.9 cm without worrisome features.
Based on ultrasound features it was decided to perform FNA on the two larger right lobe nodules. The cytology was assessed as being indeterminate (atypia) and molecular analysis using a gene expression classifier was requested. The molecular results were assessed as being suspicious for malignancy in the case of the 3 cm nodule, while in the case of the 1.3 cm nodule no result was obtained secondary to inadequate RNA in the sample.
The patient was referred for thyroid surgery, and the final pathology indicated a 0.5 cm papillary thyroid cancer in the right lobe and also three subcentimeter foci of PTC in the left lobe with findings consistent with Stage 1 differentiated thyroid cancer. After discussion it was decided to forego treatment with radioactive iodine and patient was started on suppressive doses of thyroxine.
How the Diagnosis Was Made
Thyroid nodules are very common, with 40 % of patients in their 50s having a thyroid nodule detected by ultrasound [1] and with autopsy studies reporting a prevalence ranging between 13 and 57 % [2]. As in our case, they are typically found during routine physical examination, noted by the patient or family, or incidentally diagnosed on radiological studies performed for unrelated reasons [carotid Doppler, neck/chest computed tomography (CT)]. Advances in imaging techniques and increased use of radiologic studies led to a further increase in rate of detection of thyroid incidentalomas, from about 5 % by palpation to ten times more frequently with the use of high resolution ultrasound [1, 2].
Ultrasound examination is the preferred imaging modality to evaluate newly discovered thyroid abnormalities. In one study, one in six thyroid abnormalities noted on palpation did not correspond to a defined thyroid nodule on the ultrasound. Conversely US uncovered additional non-palpable nodules over 1 cm in 27 % of patients [3]. Incidence in thyroid nodules appears to increase with age, female sex, history of iodine deficiency, and exposure to head and neck radiation [2]. Solitary nodules are about four times more common in women than in men [1].
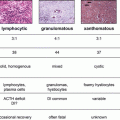
Assessing for presence of malignancy followed by presence of autonomous overactivity are the main objectives in evaluating a thyroid nodule. The incidence of thyroid cancer has been rising over the past three decades, only partly due to increase in use and performance of imaging [4]. Estimates indicate that about 5–13 % of all thyroid nodules harbor malignancy [5]. Fine needle aspiration remains the gold standard in assessing thyroid nodules for presence of cancer, however, up to 20 % of cytology results fall into the “indeterminate” category where the presence of cancer remains in doubt. A growing number of investigations over the last 10–15 years have focused on the potential use of molecular markers to confirm or exclude the presence of cancer in thyroid nodules. Several molecular tests are now available for the evaluation of thyroid nodules.
Lessons Learned
Clinical Evaluation of a Newly Discovered Thyroid Nodule
When evaluating a newly diagnosed thyroid nodule, the physician should start by obtaining a careful history with emphasis on risk factors for malignancy (exposure to radiation, family history of thyroid cancer, MEN 2a or 2b syndromes or, familial adenomatous polyposis) along with a description of nodule growth and of abnormal cervical lymph nodes if present. A review of systems should address symptoms consistent with hypo- and hyperthyroidism along with any compression related symptoms such as: dysphagia, positional dyspnea, voice changes/hoarseness, and/or cervical lymphadenopathy.
Physical examination should focus on location, size, mobility, and firmness of the nodule, presence of cervical lymphadenopathy, as well as signs of hypo- or hyperthyroidism.
Biochemical Tests
A serum thyroid-stimulating hormone (TSH) should be obtained. A TSH in the upper half of normal range or above has been correlated with increased risk of thyroid malignancy [6]. If suppressed, an elevated free thyroxine would confirm hyperthyroidism, and radioactive iodine uptake and scan are indicated as to determine the presence of a toxic/hyperfunctioning nodule or nodules [6]. In the case of hyperfunctioning nodules, fine needle aspiration (FNA) is not typically recommended as the risk of malignancy is extremely rare, although in the face of concerning ultrasound characteristics FNA may be appropriate in select cases. Measurement of serum thyroglobulin levels is not recommended as it is not specific for thyroid cancer in the presence of an intact thyroid and can be elevated secondary to benign conditions such as thyroiditis. A consensus does not exist with respect to routine measurements of unstimulated serum calcitonin levels for the evaluation of thyroid nodules. Selective use in case of family history of thyroid cancer or family history of MEN syndromes is indicated. Although the two-site, two step chemiluminiscent immunometric assays used currently are much less susceptible to interferences, they still display significant inter assay variability, and heterophilic antibodies have been described to cause falsely elevated, and occasionally low, calcitonin levels. However, it is generally accepted that values above 100 pg/mL are suggestive of medullary thyroid cancer, in which case surgery is indicated [6].
Ultrasound Characteristics and Malignancy Risk
All newly diagnosed or suspected thyroid nodules should be evaluated by ultrasound, as it provides the most accurate imaging technique in evaluating the structure of the thyroid gland. In the case of substernal extension, additional CT evaluation is appropriate.
A thyroid nodule typically represents thyroid tissue that has become distinct from surrounding parenchyma. Nonthyroidal entities can also appear as thyroid nodules such as: metastases from other primary malignancies, sarcoma, lymphoma, and intrathyroidal parathyroid. Likewise, chronic lymphocytic thyroiditis can appear on ultrasound as hyper- or hypoechoic areas separated by fibrotic strands, which may be mistakenly diagnosed as nodules. These areas also called pseudonodules do not have a halo or a sharp border and are not visualized in both longitudinal and transverse views.
Thyroid ultrasound should document the appearance and size of the thyroid lobes and any nodules or cysts and abnormal cervical lymph nodes, if noted. Sonographic features of thyroid nodules include: size (AP, transverse, and longitudinal diameter), shape (round, taller than wide), margins (borders/halo), echogenicity (hypo-, iso-, or hyperechoic), echostructure (homogenous vs. mixed solid/cystic vs. spongiform), presence of calcifications and internal vascularity. Several studies focused on identifying associations between certain US characteristics and malignancy, as summarized in Table 15.1. As seen, certain ultrasound features can be suggestive, but not definitively characterize a thyroid nodule as malignant or benign, for this purpose the gold standard remains FNA.
Table 15.1
Thyroid nodule ultrasound features
Ultrasound feature of the thyroid nodule | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) |
|---|---|---|---|---|
Microcalcifications | 26–59 | 85–95 | 24–77 | 42–94 |
Irregular borders | 17–78 | 39–85 | 9–60 | 39–98 |
Increased intranodular vascularity | 54–74 | 79–81 | 24–42 | 86–97 |
Solid | 69–75 | 52–56 | 16–27 | 88–92 |
Hypoechogenicity | 26–87 | 43–94 | 11–79 | 68–94 |
Taller than wide (transverse view) | 33–40 | 91–93 | 68–77 | 67–75 |
The size of the nodule does not predict risk of malignancy in a linear fashion. In a recent study including over 7,300 thyroid nodules the incidence of malignancy was 10.5 % in those measuring 1–1.9 cm and 15 % in thyroid nodules over 2 cm, incidence that remained relatively stable for sizes above 2 cm. Histopathology however, did change significantly, with follicular and Hurthle cell cancers being notably more frequent as the nodules got larger [9]. Micronodules (less than 1 cm) have a reported malignancy rate ranging between 2.1 and 7 % [10]. The American Thyroid Association (ATA) generally recommends biopsy of the nodules 1 cm and larger in size, with FNA of micronodules reserved for cases with high risk history, suspicious ultrasound findings or abnormal cervical lymph nodes [6].
Shape of the nodule was also assessed as predictor of malignancy; a taller than wide shape being found significantly specific for malignancy however with a low sensitivity.
Thyroid nodules may have either smooth or irregular margins. Most benign nodules will appear as well defined sonographically, while a blurred contour may indicate malignancy. The halo represents compressed perinodular blood vessels and appears as a thin iso- or hypoechoic rim surrounding usually a benign nodule. Absence of halo or a thick irregular halo (>2 mm) is, however, suggestive of follicular neoplasm [11, 12].
Echogenicity of a nodule describes its brightness compared to normal thyroid parenchyma. Solid nodules can therefore be hypo-, iso-, or hyperechoic. Hypoechogenicity has been associated with an increased risk of malignancy, although this feature is not specific [5]. Cystic fluid is anechoic. It may contain bright echogenic spots with reverberation artifact (“comet tail”) which represent colloid crystal aggregates and signify a benign character. Pure thyroid cysts are rare; most thyroid nodules are complex, having both solid and cystic components. A particular mention should be made of “spongiform” or “honeycomb” nodules, which consist of multiple cystic areas, separated by septae, and are considered to be benign.
Calcifications can be divided in microcalcifications and macro (coarse) calcifications. Microcalcifications are echogenic foci without acoustic shadowing, considered to be the equivalent of psammoma bodies on histology and highly suggestive of papillary thyroid cancer. Coarse (eggshell) calcifications are associated mainly with benign disease; however, disruption of rim calcification may be seen in cases of malignancy [13].
Thyroid nodule vascularity is most commonly evaluated by Color Flow Doppler (CFD). In case of nodules with slow intranodular flow, Power Doppler, which is independent of velocity, can be used to better characterize internal vascularity. Several studies indicate that increased internal flow increases the probability that a thyroid nodule is malignant [11, 14, 15]. One suggested classification describes the pattern of nodular flow, as absent (grade I), perinodular (grade II), and peri and intranodular (grade III) [11]. In a study on over 200 thyroid nodules, Frates et al. [15] reports the nodule vascularity as absent (0), minimal internal flow without peripheral ring (1), peripheral ring of flow (defined as >25 % of the nodule’s circumference) but minimal or no internal flow (2), peripheral ring of flow and small to moderate amount of internal flow, (3) and extensive internal flow with or without a peripheral ring (4).
Thyroid nodules that are hard to palpation have been recognized to have a higher malignant potential. Ultrasound elastography assesses tissue stiffness, by measuring the degree of distortion when a tissue is subjected to external pressure. It has been initially developed to evaluate other tissues including breast and prostate nodules, liver fibrosis and lymph nodes for malignancy. In recent years elastography has been used in evaluation of thyroid nodules and emerging data suggest that, it could represent an independent predictor for thyroid nodule malignancy, to be used in conjunction with conventional ultrasound techniques [16].
Indications for FNA Sampling: Interpretation of the FNA Results—Role of Molecular Analysis
ATA 2009 guidelines summarize indications for biopsy of thyroid nodules based on various levels of evidence. Indications for FNA are based on a combination of patient’s clinical history (e.g., exposure to radiation or personal or family history of thyroid cancer) and nodule size and appearance [6]. For example, a 1.5 cm nodule with increased intravascular flow and irregular margins would be favored for biopsy over a 2.5 cm spongiform nodule. Similarly, a 1.8 cm solid hypoechoic nodule with microcalcifications would take priority over a spongiform nodule (honeycomb appearance) 2 cm in size. Current guidelines recommend against FNA of completely cystic nodules.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree