Antibodies
Learning Objectives
• Describe the basic structure of antibodies
• Discuss the function of the variable and hypervariable portion of antibodies
• Identify the functions of the C H1–C H3 regions
• Recognize the definition of the complementarity-determining regions (CDRs)
• Compare and contrast antibody affinity and avidity
• Compare and contrast the structure and antigen-binding capability of Fab and F(ab’)2
• Describe the usefulness of Fab fragments in treating drug overdoses
• Compare and contrast the structure and function of immunoglobulin M (IgM) and IgG
• Identify the structure and biologic functions of IgG subclasses
• Identify the clinical implications of IgG subclasses
• Compare and contrast the structure and function of IgA and secretory IgA (sIgA)
• Understand the mechanism used to transport IgA to the exterior
• Understand the role of mucosal-associated lymphoid tissue (MALT) and sIgA in vaccination
• Relate the structure and function of IgD
• Restate the biologic function of IgE
• Identify the definition of an anamnestic response
• Understand the mechanisms involved in the generation of an anamnestic response
• Understand the role of cytokines in isotypic switching
• Discuss the immunologic defects in transient hypogammaglobulinemia of infancy (THI)
• Discuss the immunologic defects associated with common variable immunodeficiency (CVID)
• Identify symptoms and immunologic defects associated with hyperimmunoglobulinemia E (Job syndrome)
• Describe the drugs used to treat Job syndrome
• Understand the pathophysiology of multiple myeloma
• Describe Bence Jones proteins
• Identify the drugs used to treat multiple myeloma
• Understand the pathophysiology of Waldenström’s macroglobulinemia
Key Terms
Affinity
Avidity
Anamnestic response
Bence Jones proteins
Complementarity-determining regions (CDRs)
Fab
F(ab’)2
Fc
IgM
IgG
IgA
IgD
IgE
Isotypes
Multiple myeloma
Waldenström’s macroglobulinemia
Introduction
Antibodies are soluble proteins produced by plasma cells. They are normally found in peripheral blood and external body fluids such as saliva, tears, and colostrums. Antibodies neutralize viruses or mark antigens or microbes for destruction by phagocytosis or complement lysis. A number of different synonyms for antibodies exist. When serum is placed in an electrical field, blood proteins migrate at different rates, depending on size and charge. Small-molecular-weight albumins migrate rapidly, whereas globulin fractions migrate more slowly. Antibodies are localized in the slow-migrating gamma (γ) fractions and are termed gammaglobulins. Ultra-centrifugation also can be used to separate immunoglobulins on the basis of size. On the basis of the sedimentation rate (Svedberg units) in a centrifugal field, antibodies are divided into three different molecular weights: (1) the 7S antibody fraction with a molecular weight of 150,000, (2) the 11S fraction with a molecular weight of 300,000, and (3) the 19S fraction with a molecular weight of 900,000.
Antibody Structure
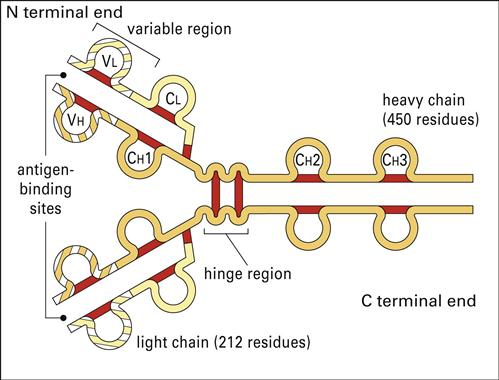
Antibodies have a basic structure which consists of pairs of heavy (H) and light (L) chains. Each heavy chain has a molecular weight of 50,000 Daltons. The weight of light chains is approximately 25,000 Daltons. Disulfide bonds link heavy chains and attach light chains to heavy chains. Heavy and light chains consist of several different globular domains that have constant or variable amino acid sequences (Figure 9-1).
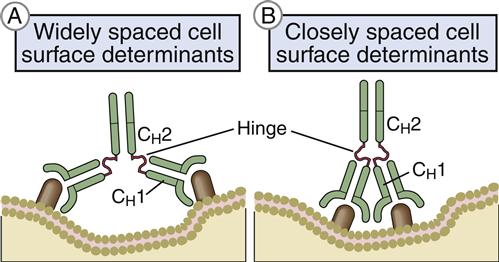
Heavy-chain constant domains are called constant regions (CH1, CH2, and CH3). Heavy-chain CH1 segments are linked to the variable (VH) domain, which is part of the antigen-combining site. The CH2 region is the hinge region that ensures antibody flexibility—the larger the hinge region, the more flexible is the antibody (Figure 9-2). Flexibility is essential because epitopes are often widely spaced on protein molecules or microbes. Attachment to cellular receptors is facilitated by the antibody CH3 region. However, some antibody subpopulations express an additional heavy-chain region (CH4) that restricts binding to select cells.
Light chains attached to the heavy chains also have constant (CL) and variable region (VL) domains. The VL domain contributes to antigen binding. These 24-kiloDalton (kDal) light chains are covalently linked to heavy chains via disulfide bonds.
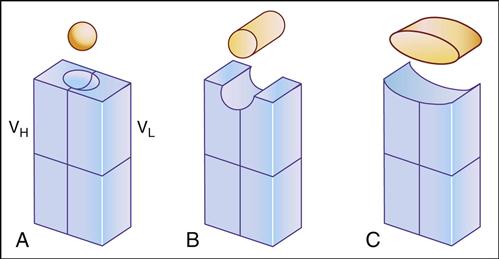
Antibody specificity is determined by the association of V regions from both heavy and light chains. In essence, V segments form a three-dimensional pocket that is the mirror image of the antigen that elicited its production (Figure 9-3).
Within the antigen-binding pocket, small 10-amino-acid hypervariable regions determine specificity at the molecular level. Amino acid sequences that form the three-dimensional antigen pocket are termed complementarity-determining regions (CDRs). Each heavy- and light-chain variable region contains three CDRs.
Affinity and Avidity
Antibody binding is described in terms of affinity and avidity. The binding strength or affinity is the result of the interaction between an antibody and a single antigenic determinant. From a practical perspective, antibody affinity is important in determining the rate at which an infection is terminated. Antibodies with high affinity will tightly bind lower concentrations of microbes and quickly terminate an infection. Low-affinity antibodies are most efficient when large antigen concentrations are present, which means that it takes longer to terminate the infections. Antibody avidity is dependent on the number of antibody-combining sites and the number of epitopes in a single antigen. It is the combined strength of multiple interactions between antibodies and epitopes. Avidity will always be geometrically higher than the affinity.
Antibody Fragments
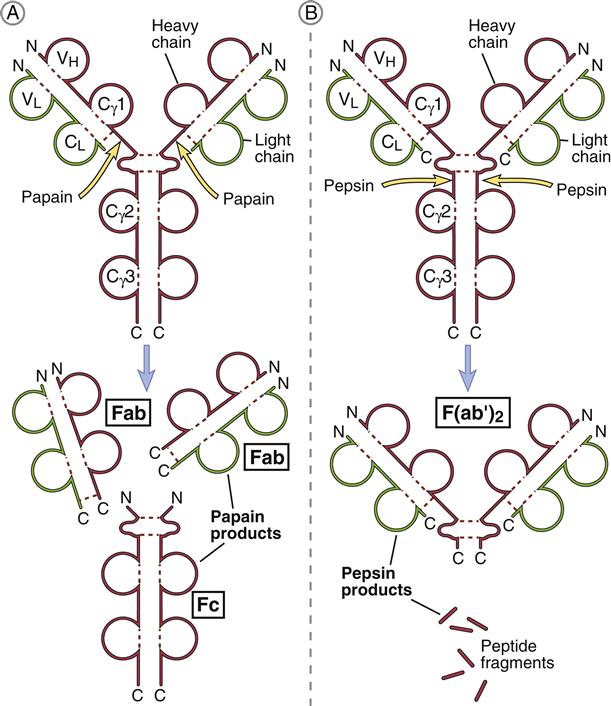
Porter delineated antibody structure by digesting antibodies with proteolytic papain and pepsin. Papain cleaves the heavy-chain CH2 domains above the disulfide bonds that connect heavy chains and yields three different fragments. The resultant pieces are identical in structure and consist of constant and variable regions of heavy (VH and CH1) and light (VL and CL) chains linked by disulfide bonds. These two fragments, each having a molecular weight of 60,000, are called Fab (fragment antigen binding). Each Fab binds a single antigen. The remaining fragment, which consists of CH2 and CH3 heavy-chain units, is easily crystallized and is called Fc (fraction crystallized). Later studies showed that the Fc portion of antibodies binds to receptors (FcR) on immunocompetent cells (Figure 9-4).
Pepsin cleaves IgG heavy chains at a point below the disulfide bond that links two heavy chains. The resulting fragment consists of two Fab linked together by heavy-chain disulfide bonds. The large-molecular-weight fragments are called F(ab’)2. Each F(ab’)2 can bind two antigens. The remainder of the antibody is reduced to small peptide fragments that have no biologic function.
Clinical Usefulness of Fab Fragments
Fab fragments are used in the clinic to treat select drug overdoses and envenomations. For example, Fab fragments are commonly used to treat digoxin overdoses or North American Crotalidae (rattlesnake) envenomations. Fab fragments neutralize the drug or the venom in blood, and the resulting equilibrium shifts away from target cell binding. Since the molecular weight of the antigen–Fab complexes are usually less than 65,000 molecular weight (MW), they pass through the kidneys and are eliminated in urine. Patients usually begin to recover within 30 minutes of receiving a bolus of Fab fragments.
Antibody Isotypes
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree