| Think anthrax |
|---|
| Employment history: herdsman, veterinarian, textile worker, hide/wool worker |
| Chest x-ray findings: widened mediastinum; pleural effusion (hemorrhagic) |
| Cutaneous lesions: necrotizing ulcer with bullae; eschar (bacteria present beneath lesion) |
| Hemorrhagic meningitis: gram-positive bacteria present in CSF |
Pathogenesis of anthrax
Animal studies demonstrate that spores introduced through the skin rapidly germinate and within hours the subcutaneous lesions swell and numerous encapsulated gram-positive rods, often “box-car” in shape, are present. The capsule is composed of poly-D-glutamic acid and probably protects the bacterium from host recognition and attack by neutrophils (it is thought to be anti-phagocytic). It is a bona fide virulence factor carried on a plasmid, pX02, and loss of the plasmid renders the bacterium less virulent (Table 125.2). The successful animal vaccine developed by Max Sterne uses a strain cured of pX02. Wildtype B. anthracis possesses a second plasmid, pX01, that controls toxin production. The toxin consists of three proteins, protective antigen (PA), edema factor (EF), and lethal factor (LF), typical binary toxins with an enzymatic site and binding site. After proteolysis of a small fragment from PA, the protein binds to six other PA fragments forming a heptameric channel to which LF and EF competitively bind and are then endocytosed. EF leads to increased levels of cyclic AMP and swelling whereas LF leads to cell death (Figure 125.1).
| Zoonotic disease | Differentiating points |
|---|---|
| Tularemia (Francisella tularensis) | Prominent lymphadenopathy |
| Orf | Painless bullae |
| Cat scratch | Fleeting eschar |
| Tick-borne Rickettsia: R. africae, STARI, RMSF | Small eschar, often with body rash |
| Plague | Lymphadenopathy; Four Corners area of the United States |
Abbreviations: STARI = Southern tick-associated rash illness; RMSF = Rocky Mountain spotted fever.
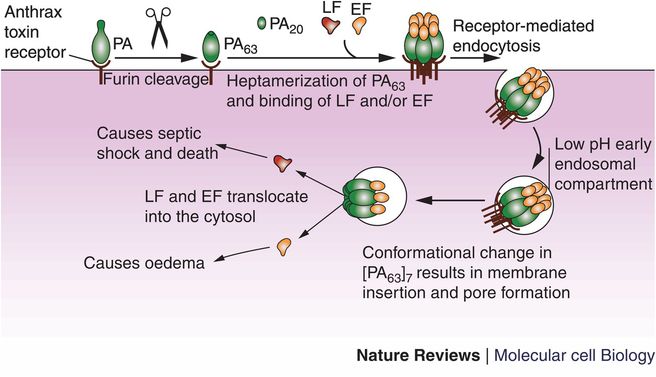
Figure 125.1 Protective antigen (PA) is secreted by Bacillus anthracis bacilli and binds to cellular anthrax toxin receptor. A 20-kDa fragment of PA is cleaved by furin, the resulting PA63 fuses with six other PA63 fragments forming a pore for the entry of EF and LF. The structure is brought into the cytosol in an endosome where EF and LF then translocate from the endosome into the cytosol causing edema and cell death, respectively.
Spores inhaled into the lung reach the alveoli where they are phagocytosed by macrophages. Macrophages then transit through the lymphatic system. Germination of the spores may occur up to 60 days following inhalation as shown in monkeys. In gastrointestinal anthrax hemorrhage and edema are located in the intestinal wall. In some animals the M cell in Peyer’s patches may be the site of entry of the microorganism.
Clinical manifestations of anthrax
The clinical presentation of anthrax is variable and dependent on the route of exposure. Presentations include cutaneous, inhalational, and enteric (gastrointestinal and oropharyngeal) forms, which may result in disseminated disease involving the central nervous system (meningitis) and secondary bacteremia leading to sepsis. The majority of infections are due to cutaneous disease and these infections have the lowest mortality. Cutaneous lesions referred to as eschars develop as single or multiple lesions after exposure to B. anthracis spores. Spores enter breaks in the skin and following an incubation period ranging from several hours to 3 weeks the inoculum site develops into a papule followed by a ring of vesicles, accompanied by edema, regional lymphadenopathy, and necrosis. The lesion then forms the typical dark eschar, which is accompanied by profound edema usually from 5 to 7 days. Resolution occurs over a period of several weeks. Although the eschar is usually not painful most patients may experience constitutional symptoms or may present with sepsis especially with lesions involving the face, neck, and upper chest. Infection involving the gastrointestinal tract occurs after ingestion of meat from animals dying of anthrax. The disease has an incubation period of 1 to 6 days and the presentation may initially include mild symptoms such as fever, nausea, vomiting, and mild diarrhea but then progresses to more severe symptoms of bloody diarrhea, acute abdominal pain, hematemesis, and ascites. Complications include obstruction, perforation, sepsis, and death. Pathologic evaluation demonstrates ulcerating, necrotizing lesions accompanied by regional lymphadenopathy. Inhalation anthrax is a rare but dangerous form of the disease, which has public health implications due to its association with bioterrorism. Infection involves lymph nodes rather than lung parenchyma, which makes the term “pulmonary” a misnomer since complications result from germinating spores transported to the lymphatic system by macrophages. The disease has a biphasic presentation with nonspecific initial symptoms of fever, headache, chills, malaise, nonproductive cough followed by a more rapid progressive form when patients experience dyspnea, cyanosis, respiratory failure, sepsis, and death. The incubation period ranges from 4 to 11 days. The chest x-ray findings most consistent with inhalation anthrax include mediastinal widening, pleural effusions, and the presentation of hyperdense necrotic-appearing lymph nodes involving the mediastinum on computerized tomography. Infection of the central nervous system is a life-threatening complication of any of the major forms of anthrax and carries a poor prognosis. In contrast to common causes of bacterial meningitis, the cerebral spinal fluid contains large numbers of red blood cells, indicating a hemorrhagic component (Table 125.3).
| Virulence factor | Contribution to disease |
|---|---|
| Plasmid X02 | Encodes for poly-D-glutamic acid capsule which may mask bacterium from host defenses |
| Plasmid X01 | Encodes for tripartite toxin complex: protective antigen, lethal factor and edema factor |
| Spore | Survival of infectious propagule for up to ~90 years in conducive soils; exosporium allows for delay of germination within alveolar macrophages |
Treatment of anthrax
Treatment for inhalational anthrax includes ciprofloxacin or doxycycline plus consideration of one or two other antimicrobials, such as rifampin, vancomycin, imipenem, chloramphenicol, penicillin or ampicillin, clindamycin, and clarithromycin. Doxycycline may be less optimal for cases with meningitis because of poor central nervous system penetration. For cutaneous anthrax, ciprofloxacin or doxycycline are recommended for initial therapy. Duration of combined intravenous and oral therapy should continue for 60 days for all anthrax cases because of the potential persistence of spores after an aerosol exposure. For additional details of antimicrobial regimens, see Chapter 122, Bioterrorism, Table 122.4. A new modality of therapy is the use of a monoclonal antibody (raxibacumab) that inhibits PA binding to anthrax toxin receptors.
Novel therapeutics
Nonantimicrobial therapeutics targeting various stages of anthrax infection are the subject of intense investigation since the disease carries a high mortality. Medications that have been approved for other conditions have been under investigation. These include but are not limited to amiodarone, verapamil, nifedipine, statins, celecoxib, dantrolene, N-acetyl-L
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree





