Variable (adults)
Normal values
Red blood cells count (/ml)
Male 4.5–5.9 × 106
Female 3.5–5.0 × 106
Hematocrit (%)
Male: 39–49
Female: 33–43
Blood hemoglobin level (Hb)
Male: 13–17 g/dl
Female: 13–15 g/dl
Reticulocyte count (%)
0.8–2.5
Mean corpuscolar volume (MCV)
85–100 fL
Mean corpuscolar Hb concentration (MCHC)
31–35 g/dl
Mean corpuscolar Hb (MCH)
28–33 pg/cell
Serum transferrin
200–300 mg/dl
Serum Fe
75–160 mcg/ml (m) 60–150 mcg/ml (f)
Serum Ferritin
20–300 ng/ml (m) 20–120 ng/ml (f)
Serum haptoglobin
50–220 mg/dl
Vitamin B12
200–1,000 pg/ml
Folate
2–10 ng/ml
3.2 The Kinetics of Red Blood Cells
RBC are generated in the bone marrow under the influence of erythropoietin (EPO) and their production requires a number of factors, including zinc, iron (Fe), vitamin B12, folate, tyrosine, androgen hormones, and cortisol [4]; the basal release of RBC is 15–20 ml/day but this rate can decuplicate during acute anemia provided that the iron stores are repleted and in the presence of a normal renal function [5]. As the mature RBC are devoid of mitochondria as well as of intrinsic reparatory mechanisms, their ageing-related decrease of energy levels is associated with changes of the membrane properties, including the reduction of their fluidity and deformability and the increase of density and viscosity; all these changes ultimately lead to their removal from circulation and destruction in the spleen and in the reticuloendothelial system (RES) [6–8]. In normal conditions, the overall life span of RBC is 120 days. Other processes responsible for their anticipated elimination from the bloodstream include the premature death of mature RBC (eryptosis) and the removal of RBC just released from the bone marrow (neocytolisis). Both mechanisms are responsible for the maintenance of an appropriate circulating mass of RBC and are inhibited by EPO [5].
3.3 The Physiological Consequence of Acute Anemia
Basically, Hb plays a dual role. First, as RBC carry O2 from the lungs to the cells, according to the formula:
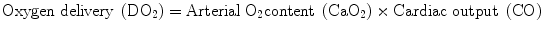 it appears that a reduction of the CaO2, which is mainly determined by the total Hb and its O2 saturation (SaO2), being negligible, the amount of O2 dissolved in the plasma in normobaric conditions sets the stage for a reduced O2 availability to the tissues with the subsequent onset of anaerobic metabolism [9, 10]. Second, since Hb scavenges CO2 from the cells to the lungs, its drop is associated with the increase of the tissue CO2 content. In a resting healthy organism, a number of mechanisms can counterbalance an acute isovolemic reduction of Hb to as low as 5 g/dl [11]; these include (a) the leftward shift of the Hb dissociation curve determining a facilitated download of O2 toward the cells, leading to an increased extraction of O2 (O2ER); (b) a compensatory tachycardia and tachipnea; and (c) the concomitant increase of the heart rate (HR), stroke volume (SV), and CO driven by the hypoxia-induced increased production of catecholamines. However, if the anemia aggravates and/or in the presence of concomitant limited cardiac and respiratory reserves, all these adaptative mechanisms become exhausted and tissue respiratory and metabolic acidosis ensue due to the contemporaneal increase of CO2 and of the lactate produced under anaerobic conditions [4, 9, 12]. Moreover, it has become clear that the tissues are not equally vulnerable to a reduced O2 availability and marked differences exist in terms of O2ER capabilities among different organs and sometimes also within the same organ [12].
it appears that a reduction of the CaO2, which is mainly determined by the total Hb and its O2 saturation (SaO2), being negligible, the amount of O2 dissolved in the plasma in normobaric conditions sets the stage for a reduced O2 availability to the tissues with the subsequent onset of anaerobic metabolism [9, 10]. Second, since Hb scavenges CO2 from the cells to the lungs, its drop is associated with the increase of the tissue CO2 content. In a resting healthy organism, a number of mechanisms can counterbalance an acute isovolemic reduction of Hb to as low as 5 g/dl [11]; these include (a) the leftward shift of the Hb dissociation curve determining a facilitated download of O2 toward the cells, leading to an increased extraction of O2 (O2ER); (b) a compensatory tachycardia and tachipnea; and (c) the concomitant increase of the heart rate (HR), stroke volume (SV), and CO driven by the hypoxia-induced increased production of catecholamines. However, if the anemia aggravates and/or in the presence of concomitant limited cardiac and respiratory reserves, all these adaptative mechanisms become exhausted and tissue respiratory and metabolic acidosis ensue due to the contemporaneal increase of CO2 and of the lactate produced under anaerobic conditions [4, 9, 12]. Moreover, it has become clear that the tissues are not equally vulnerable to a reduced O2 availability and marked differences exist in terms of O2ER capabilities among different organs and sometimes also within the same organ [12].
3.4 Causes of Anemia in the Critically Ill Patient
Similarly to other fields of medicine, either a reduced production of RBC and/or a decrease of their life span account for the occurrence of anemia in critically ill patients [4]; actually, both mechanisms can act simultaneously in many conditions commonly encountered in the ICU independently from the cause of admission, including advanced age, poor nutritional condition, recent surgical procedures, unresolved inflammatory conditions, etc. In these circumstances, a severe anemia can develop in 1 week from the onset of the disease, requiring the ICU admission [3].
3.4.1 Anemia Due to a Reduced Production of RBC
Albeit a reduced production of Hb can be caused by several factors, those more frequently encountered among patients admitted to the ICU include:
(a)
Persisting inflammatory conditions, not only determined by chronic conditions such as neoplasms, vasculitides, and rheumatologic conditions but also by unresolving sepsis, post-operative states, etc. [13]. These conditions appear particularly relevant as more and more elderly subjects survive the initial insult determining their admission to the ICU, only to become chronic critically ill patients who cannot be weaned from the mechanical ventilation [1]. In these circumstances, several inflammatory and counter-inflammatory mediators produced during either the initial or the more advanced phase of their disease, including Tumor Necrosis Factors-α (TNF- α) and Interleukin-1 (Il-1) and Il-6, negatively reduce Fe metabolism and impair the feedback existing between its enteric adsorption and the body stores [4]; this latter phenomenon is aggravated by an increased production of hepcidin, a protein synthetized in the liver, which also inhibits the release of the Fe stored into the RES. At the same time, since the production of EPO is inappropriately reduced even in the presence of abnormally low values of Hb and the number of its receptors on the target cells is decreased, the response of the bone marrow is blunted.
In the aforementioned circumstances and in the absence of other confounding factors, the anemia is mild to moderate, with Hb > than 8 g/dl, and with a normal mean cell volume (MCV) and mean cell hemoglobin (MCH) [4].
(b)
Fe deficiency, caused by blood loss or inadequate dietary intake. Actually, Fe-deficiency anemia is rather common, having been reported in as many as 9 % of ICU patients [14]. The classic signs of iron deficiency anemia can be difficult to evaluate in ICU patients, and the diagnosis is based on the biochemical markers of the iron metabolism (Table 3.2). As in these patients factors other than iron deficiency can determine hyposideremia, other markers must be suited to confirm the diagnosis, including:
Table 3.2
Differential features of anemia of chronic disease and iron deficiency anemia
Variable | Anemia of chronic disorders | Iron deficiency anemia |
|---|---|---|
Hb | May be ≥8 g/dl | May be ≤8 g/dl |
MCV | Normal/↓ | ↓ |
MCH | Normal/↓ | ↓ |
Serum Fe | ↓ | ↓/↓↓ |
Serum Ferritin | Normal/↑ | ↓ |
Serum hepcidin | ↑ | ↓ |
Reticulocytes | ↓ | ↓ |
(i)
Serum ferritin: Even if its value increases in the presence of sepsis and severe infections as it is an acute-phase reactant, low values are suggestive of iron deficiency.
(ii)
Serum transferrin and total serum binding capacity, which are increased during iron deficiency; in the same condition, the transferring saturation is reduced. However, it should be noted that other factors, including alcohol, neoplasm, and inflammatory conditions can decrease the sensibility and sensitivity of these markers.
(iii)
RBC zinc protoporphyrin, whose values increase during iron deficiency and is not affected by a concomitant inflammatory state.
Hematological features of anemia associated with Fe-deficiency anemia include Hb values <8 g/dl and reduced MCV and MCH [14].
(c)
Get Clinical Tree app for offline access

Vitamin B12 and folate deficiencies have been reported in 2 % of ICU patients and are associated with a reversible failure of the bone marrow causing the disturbed synthesis of DNA and megaloblastic hematopoiesis, leading to the release of RBC larger than normal [15]. In case of isolated Vitamin B12 deficiency, a demyelinating disease of the nervous system can coexist or anticipate the onset of anemia [16]. Since the enteral adsorption of Vitamin B12 requires the action of the Intrinsic Factor (IF), which is synthetized by the gastric parietal cells and of another receptor located in the distal ileum, conditions associated with gastric or enteric mucosal disuse or atrophy, extensive gastric and/or ileal resections can determine the occurrence of Vitamin B12 maladsorption and subsequent deficiency [16]. Moreover, H2 receptor antagonists, proton pump inhibitors can impair the absorption of both Fe and Vitamin B12 [17–19]. The diagnosis of Vitamin B12 deficiency can be elusive, because (a) as many as 15–25 % of patients have normal Hb and MCV [20]; and (b) the affected patients admitted to the ICU cannot report the symptoms associated with the neuropathy. However, the diagnosis should be suspected in the presence of a progressive reduction of Hb and contemporaneal increase in MCV. The diagnostic workup of Vitamin B12 and folate deficiency-related anemia should include the following [16, 20]:
(i)




The measurement of blood Vitamin B12 levels should be interpreted with caution, because, with the exclusion of extremely low values (<100 pg/ml) which are diagnostic, both false negative and false positive results have been reported, which have been ascribed to protein carrier other than cobalamin.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree




