CHAPTER 46 Anemia and Red Blood Cell Disorders
Introduction
Anemia is a common manifestation of systemic illness. The World Health Organization (WHO) estimates that the number of anemic people worldwide approaches two billion. Women and children are more commonly affected: the WHO estimates that nearly 50% of women and children from developing nations and perhaps a quarter of men are anemic.1 Anemia in immigrants can be a result of both acquired and inherited causes (Table 46.1). Unfortunately, comprehensive studies describing the incidence and etiology of anemia in immigrant populations are lacking. However, the worldwide geographic distribution of several genetically based red blood cell (RBC) disorders (thalassemia, hemoglobin C and E, ovalocytosis, and sickle cell disease), the prevalence of iron deficiency, and the geographic prevalence of other diseases affecting the hematologic system (malaria, helminth infection, HIV, TB) are well known.2,3 Therefore, understanding the prior prevalence and geographic distribution of anemia in the country of origin is helpful when evaluating a new immigrant with a hematologic problem (Box 46.1).
Table 46.1 Causes of anemia in immigrants
Box 46.1 Case study 1
Learning issues
Additional data
| Reticulocyte count: | 2.5% |
| Peripheral smear: | Hypochromic microcytes and ‘pencil-shaped cells’ |
| No fragmented or ‘bite cells’ | |
| Stool hemoccult: | Negative |
| Malarial smear: | Negative |
| Total bilirubin: | 2.1 mg/dL (normal <1.5) |
| Direct bilirubin: | Normal (elevated indirect bilirubin) |
| LDH: | 275U/L |
| Alkaline phosphatase: | Normal |
| AST/ALT: | Normal |
| Haptoglobin: | <5 mg/dL |
| Ferritin: | 30ng/mL (normal 10–150) |
| Hemoglobin electrophoresis: | Normal amounts of HbA1, and HbA2, no Hb F or HbE |
| Heinz body prep: | Negative |
Geographic Distribution of Red Blood Cell Disorders
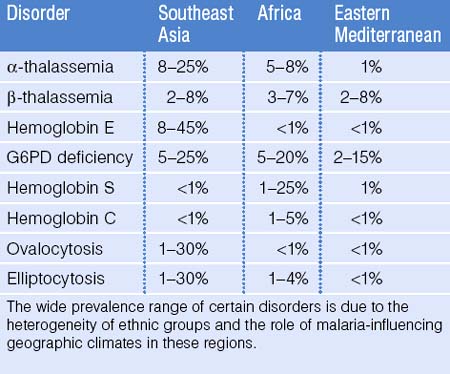
Malaria affects the tropical regions of the world and it is postulated that most genetically-based RBC disorders, such as the thalassemias, hemoglobinopathies, ovalocytosis, and elliptocytosis provide a selective protective advantage against malaria parasite infection. Indeed, malaria has had one of the most profound evolutionary effects on the human genome: genetic RBC disorders have a high prevalence in areas of the world where malaria once was or still is a major health concern.4 It is estimated that 4.83% of the world’s population carry globin gene variants, including 1.67% of the population heterozygous for α- or β-thalassemia and 1.92% carry sickle hemoglobin.5 Due to population movement and immigration, these disorders are now seen on all continents of the world. Although accurate epidemiologic data are not available for all parts of the world, it is known that both α- and β-thalassemia are more common in Africa, the Mediterranean, India, and Southeast Asia. Sickle cell disease is seen in 1–2% of infants born in tropical Africa. However, if the heterozygous state provides a selective protective advantage against malaria, the prevalence in adults may be much higher.3 Sickle cell disease is also seen to a lesser extent in India and the Middle East. Glucose 6-phosphate dehydrogenase (G6PD) deficiency is seen with varying frequency throughout Africa, Asia, and Central and South America. Hemoglobin C is most prevalent in West Africa whereas hemoglobin E is seen most exclusively in Southeast Asia. The red cell membrane defect, ovalocytosis, is more prominent in Southeast Asia whereas hereditary elliptocytosis is more common in West and North Africa. Table 46.2 provides an estimate of the frequency of RBC disorders in the three groups with the highest known prevalence: Southeast Asians, Africans, and people from Eastern Mediterranean countries.3,6–8 These estimates are a compilation of WHO data and published data from prenatal screening clinics and newborn testing series. It should be emphasized, however, that even within geographic regions, the heterogeneity of ethnic groups leads to a wide variation in the prevalence of these disorders. Indeed, an excellent example in the developed world is from Italy: the prevalence of hemoglobin disorders in Northern Italy is 1–2% but reaches 12% in Sardinia.6
Common Acquired Causes of Anemia
Iron deficiency anemia
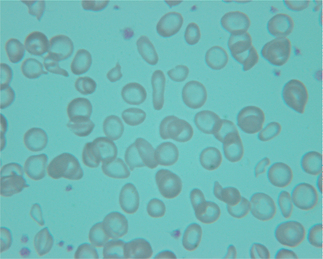
The WHO estimates that 50% of anemia worldwide can be attributed to iron deficiency; it is the most common nutritional deficiency.1 Iron balance is a reflection of dietary iron intake, adsorption, and iron (blood) loss. Iron is available in the diet as heme iron from animal protein sources and non-heme iron from vegetable sources. Heme iron is readily absorbed in the diet as is iron in breast milk. In contrast, iron from vegetable sources has low bioavailability. Due to menstruation, lactation, and iron loss during pregnancy, women of reproductive age worldwide have the highest incidence of iron deficiency. This population should be considered for supplementation. Gastrointestinal tract blood loss, the major source of iron loss, has a wide differential diagnosis. This includes peptic ulcer disease and colon cancer as in the general population but also includes helminth infection in immigrant populations. Iron deficiency leads to a microcytic anemia occasionally associated with thrombocytosis. The cells have increased central pallor and occasional elongated ‘pencil cells’ and target cells (Fig. 46.1). The red cell distribution width (RDW) is elevated and the reticulocyte count is low. Serum ferritin is characteristically low (<15 ng/mL) in uncomplicated iron deficiency and is the single best test for assessing iron stores. Since ferritin is also increased by inflammation, iron deficiency can be identified with greater sensitivity by using a higher cutoff value of ferritin of <30–100 ng/mL.9 A ratio of soluble transferrin receptor to ferritin level may also be helpful in distinguishing iron deficiency from anemia of chronic disease.
Malaria
The RBC protozoal infection, malaria, is endemic to most of the equatorial areas of the world. The prevalence of malaria in immigrants, particularly from Africa, has been reported to be as high as 15%.10 Anemia is the most common manifestation of malaria infection but leukopenia and thrombocytopenia (often from splenomegaly) may also occur. The pathophysiology of anemia is multifactorial: not only is there parasite-mediated RBC destruction and increased splenic removal, malaria infection also suppresses RBC production by the bone marrow.11 Therefore, the reticulocyte response may not be as vigorous as in a purely hemolytic process. Occasionally, malaria infection is associated with immune-mediated RBC destruction and a positive direct Coombs test due to adsorption of immunoglobulin and complement onto the RBC surface. Severe acute intravascular hemolysis may rarely occur (blackwater fever) leading to hemoglobinemia, hemoglobinuria, and severe hyperbilirubinemia. Recurrent malaria infection can be immunosuppressive and can be complicated by secondary bacterial or viral infection. Fifty percent of patients develop some degree of splenomegaly which contributes to the pancytopenia often seen in chronically infected patients.
HIV
Human immunodeficiency virus infection and AIDs can cause a plethora of hematologic problems. Early on during HIV infection, immune thrombocytopenia is common as is the development of antiphospholipid antibodies. Anemia is the most common manifestation of HIV infection and is multifactorial due to both direct and indirect effects of the virus.12 Anemia is most often a hypoproliferative, low reticulocyte anemia due to anemia of chronic disease. Often, there is a blunted erythropoietin response. Coombs-positive autoimmune hemolytic anemia also occurs with increased frequency in HIV infection. Antiretroviral therapy often causes macrocytosis.
Anemia of chronic disease
Anemia of chronic disease (ACD), perhaps best termed anemia of acute or chronic inflammation, is a hypoproliferative anemia resulting from inflammatory cytokine effects on RBC production.9 The hallmark is cytokine-induced disturbance of iron homeostasis. Iron, present in the bone marrow, is sequestered in reticuloendothelial cells and is not available for RBC and hemoglobin production. Often, ACD is a normochromic-normocytic anemia but it can progress to a microcytic anemia. The reticulocyte count is low, whereas the ferritin is characteristically >100 ng/mL and is often elevated. The erythropoietin response is blunted. Table 46.3 lists common causes of ACD.
Table 46.3 Common causes of anemia of chronic disease
Get Clinical Tree app for offline access 
|