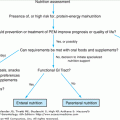
Anemia: Introduction
Anemia, a common clinical syndrome in older adults, has been the focus of renewed attention over the last decade, as attested by the increasing number of publications addressing its clinical epidemiology. Anemia in older persons tends to be multifactorial in origin, and is often associated with a combination of chronic medical conditions. The clinical presentation of anemia in older adults is complex, and symptoms are often insidious and nonspecific. Unless severe, anemia it is often perceived as a relatively benign condition that is either a normal accompaniment to aging and/or merely a marker of prevalent chronic diseases. However, data gathered to date have established that this maybe an incorrect perception. Consistently, several studies have shown that anemia, even if mild, is a strong, independent, risk factor for major adverse outcomes in older adults, including decline in physical and cognitive function, frailty, disability, and mortality. Aside from its prognostic implication, it has been hypothesized that even nonsevere anemia could causally contribute to the occurrence of these adverse outcomes, and that anemia correction could potentially lead to improved outcomes in older adults. These hypotheses remain to be proven. Future studies, including large randomized clinical trials (RCTs), are expected to contribute key insight as to whether correction of anemia that is nonsevere in the broad older population could prevent additional morbidity and/or mortality. In this context, aiming to summarize valuable information for the health professional involved with the care of older adults, this chapter presents basic information on a number of relevant issues related to the topic of anemia in older adults, including insight regarding prevalence, associations with major adverse clinical outcomes, and potential intervention opportunities.
Definition of Anemia in Older Adults
Conceptually, anemia is a clinical syndrome caused by a reduced mass of circulating red blood cells (RBCs). In practice, anemia is often operationally defined as a decreased level in any of the following parameters: the concentration of hemoglobin (Hb) in the whole blood; the proportion occupied by RBCs in a sample of whole blood—that is, hematocrit (Hct); and/or the number of RBCs in a standardized volume of whole blood. Often, Hb is expressed as g/dL, Hct as %, and RBC count as the number of RBCs in millions per microliter. Consistent with the literature, we will use Hb as the key parameter to define anemia in this chapter.
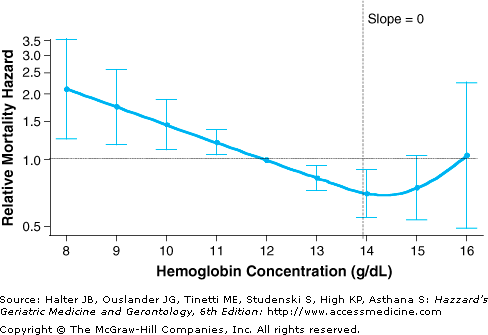
A widely used standard to define anemia in older adults is the one usually referred to as the World Health Organization (WHO) criteria, published in the 1968 WHO report on nutritional anemias. According to this criteria, anemia is defined as a Hb less than 12 g/dL in older women, and less than 13 g/dL in older men. This gender difference is primarily because of the differences in the distributions of Hb in older men and women. These criteria are the same as proposed earlier (1933) by Wintrobe. Lower Hb cutoffs have also been proposed for anemia definition in older adults, under the nonempirical argument that the concentration of Hb is “normally” lower in older populations. Important limitations of the methods used to define anemia in older adults should be acknowledged, however. Current criteria used to select the Hb cutoff for anemia have been derived primarily on the basis of statistical distribution considerations; i.e., the Hb cutoff used to separate subjects with and without anemia being the one corresponding to minus 2 standard deviations (−2 SD) below the mean calculated in an apparently healthy, standard population. Although this constitutes a useful and traditional method for defining the lower limit of the “normal range” of a biological parameter in general, it has limitations. Apparently healthy older populations, which are often defined in terms of self-reported clinical diseases, are likely to include older adults with subclinical diseases and health status changes (e.g., inflammation, decline in kidney function, etc.). These subclinical factors may shift the Hb distribution in the whole older population to the left (toward lower values). Thus, the use of −2 SD as the key criterion may result in biased, lower-than-optimal Hb cutoffs for a definition. Additionally, this statistical distribution method, which assumes a meaningful threshold difference between subjects with Hb levels below −2 SD (abnormally low) versus those with Hb levels between −2 SD and +2 SD (“normal” range), does not take into account the direct relationship between Hb and the risk of clinically relevant outcomes. In fact, recent observational epidemiologic studies have demonstrated that Hb is related to the risk of major adverse clinical outcomes in a rather continuous, curvilinear pattern (see “Outcomes Associated with Anemia”). The observed pattern was marked by an association of highest risk of outcomes with lowest Hb levels, and a monotonically risk decrement with increasing Hb levels up to mid-normal Hb levels (Figure 102-1). Even within the “normal range,” a risk gradient was observed, with Hb levels considered per current standards as low-normal being associated with a higher risk of outcomes such as frailty, disability, and mortality, as compared to levels currently perceived as mid-normal Hb. Consistently, analysis of the relationship between Hb and serum erythropoietin in older women revealed that that serum erythropoietin levels were significantly higher at low-normal Hb, as compared to mid-normal Hb, where serum erythropoietin levels were lowest (Figure 102-2). Collectively, current evidence suggests that while the WHO criteria constitute a valid approach to define data in older adults, it may not be optimal, at least from the risk prediction perspective.
Figure 102-1.
Relationship between hemoglobin (Hb) concentration and 5-yr all-cause mortality in community-dwelling disabled older women (Women’s Health and Aging Study I, 1992–2000). The figure shows a graphical display of relative risk estimates for the mortality linked to specific Hb concentrations compared with the risk linked to Hb of 12 g/dL. The curve represents smoothed relative mortality hazards across Hb concentrations, and the bars indicate their 95% confidence intervals. Also indicated is the 13.9 g/dL Hb threshold at which the slope of mortality risk decline was no longer statistically significant (i.e., the 95% confidence interval for the slope of the tangent included 0). The y-axis was transformed so that, for example, the graphical display of an increase in risk of the magnitude of two (hazard ratio (HR) = 2) would be equivalent to that of a decrease in risk of the same magnitude (HR = 0.5) in terms of scale size. Risk estimates are adjusted for age, race, congestive heart failure, coronary heart disease, peripheral artery disease, stroke, pulmonary disease, cancer, estimated creatinine clearance, ankle–arm index, depression, body mass index, smoking status, education, disability performing basic activities of daily living, and number of domains with difficulty. (Chaves PHM, Xue Q-L, Guralnik JM, et al. What does constitute normal hemoglobin concentration in community-dwelling disable older women? J Am Geriatr Soc. 2004;52(11):1811–1816. Modified, reprinted with permission.)
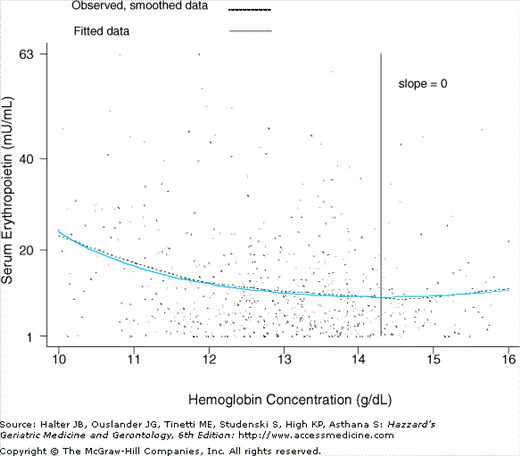
Figure 102-2.
Graphical display of the curvilinear relationship between serum erythropoietin and hemoglobin (Hb) concentration in community-dwelling disabled older women (Women’s Health and Aging Study I, 1992–1995). The slope of the tangent line to the EPO versus the Hb curve was equal to zero at the Hb threshold point of 14.3 (95% CI = 13.2–15.4). EPO at a Hb concentration of 12 g/dL was 28% greater than EPO at a Hb concentration of 13.9 g/dL (95% CI = 15–44%). Adjustment for age, race, and creatinine clearance did not change this difference estimate. Similarity between observed (smoothed) and fitted curves indicated proper model fit. (Chaves PHM, Xue Q-L, Guralnik JM, et al. What does constitute normal hemoglobin concentration in community-dwelling disable older women?J Am Geriatr Soc. 2004;52(11):1811–1816. Modified, reprinted with permission.)
Arguments have been made in favor of the use race-specific criteria for anemia definition. This perspective is supported by a recent observational study conducted in the Health, Aging, and Body Composition Study. In that study, although concerns with residual confounding by comorbidity burden as well as with selection bias at least partially explaining study findings cannot be discarded, race was shown to be an effect modifier of the associations of WHO-defined anemia with all-cause mortality (over 6 years) as well as incident mobility disability (over 4 years); that is, anemia was associated with an increased risk of mortality and disability in white, but not in black older adults.
Finally, it should be acknowledged that what may constitute best naturally occurring levels of Hb in observational studies of older adults from a risk prediction perspective does necessarily constitute optimal levels from other clinical decision-making perspectives. In fact, major discrepancies have been reported between Hb levels linked to lowest risk of adverse outcomes in older adults in observational studies with “optimal” target Hb of recombinant erythropoietin in RCTs (see “Therapeutic Considerations”).
Prevalence and Types of Anemia
The prevalence of anemia in older adults varies according to the setting in which it is estimated. Higher prevalence estimates have been reported in long-term care facilities such as nursing homes than in the general community-dwelling older population. This difference maybe explained by the fact that, in general, anemia is associated with poorer health status. Differences in prevalence estimates within similar settings have also been reported, and maybe primarily attributed to methodological differences, such as variations in background characteristics of targeted populations, response rates, and the way anemia was defined.
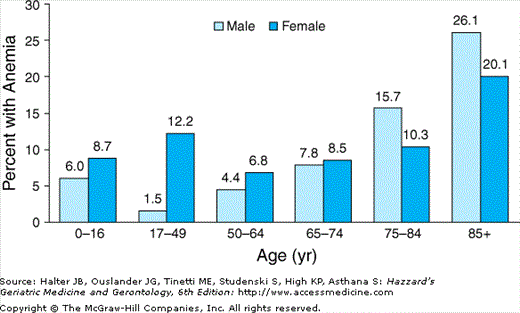
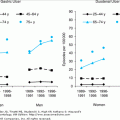
A recent study reported on the prevalence of anemia in the large U.S.-representative sample of noninstitutionalized older adults who participated in the Third National Health and Nutrition Examination Survey (NHANES III, 1988–1994). In that study, the prevalence of anemia was 11% in older men and 10.2% in older women. Overall, the prevalence in the population of community-dwelling subjects 65 years and older was 10.6%. There were, nonetheless, significant differences according to age groups (Figure 102-3). In both men and women 65 years and older, the prevalence of anemia steadily increased with advancing age, being highest in the 85+-year age group. In subjects who were 65 to 74 years old, the prevalence of anemia was slightly higher in women than in men; however, in the age groups 75 to 84 years and 85+ years old, the prevalence of anemia was substantially higher in men than in women.
Figure 102-3.
Percentage of persons with anemia according to age and sex (National Health and Nutrition Examination Survey (NHANES) III, phases 1 and 2, 1988–1994). (Guralnik JM, Eisenstaedt RS, Ferrucci L, et al. Prevalence of anemia in persons 65 yrs and older in the United States: evidence for a high rate of unexplained anemia. Blood. 2004;104:2263–2268.)
As compared to the distribution of Hb in older men, anemia in older women was shifted to the left; that is, toward lower values (Figure 102-4). Only a very small minority of anemia in community-dwelling older adults is severe, with less than 1% of the total sample having Hb levels lower than 10 g/dL (see Figure 102-4). Additionally, in that study, significant differences in the prevalence of anemia according to race and ethnicity were reported (Figure 102-5). The prevalence of anemia was lowest in non-Hispanic whites, and highest in non-Hispanic blacks. Anemia prevalence in Mexican Americans was only slightly higher than that of non-Hispanic whites.
Figure 102-4.
Distribution of hemoglobin in community-dwelling persons 65 yrs and older according to sex (National Health and Nutrition Examination Survey (NHANES) III, phases 1 and 2, 1988–1994). (Guralnik JM, Eisenstaedt RS, Ferrucci L, et al. Prevalence of anemia in persons 65 yrs and older in the United States: evidence for a high rate of unexplained anemia. Blood. 2004;104:2263–2268.)
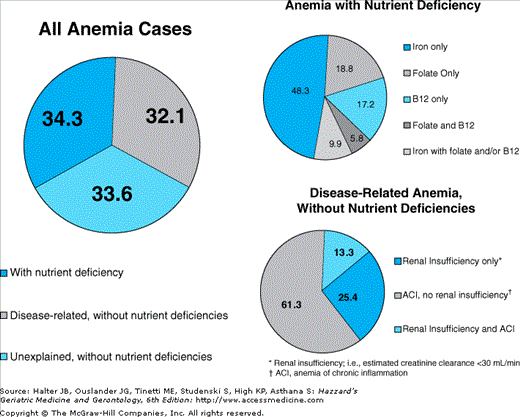
Figure 102-5.
Distribution of types of anemia in community-dwelling persons 65 yrs and older (National Health and Nutrition Examination Survey (NHANES) III, phases 1 and 2, 1988–1994). (Guralnik JM, Eisenstaedt RS, Ferrucci L, et al. Prevalence of anemia in persons 65 yrs and older in the United States: evidence for a high rate of unexplained anemia. Blood. 2004;104:2263–2268.)
Anemia maybe classified in a number of different ways. A practical scheme used in the geriatrics literature involves the classification of anemia in the following major categories: (a) nutrient deficiency, (b) anemia of chronic kidney disease (CKD), (c) anemia of chronic inflammation (ACI; previously referred to as anemia of chronic disease), and (d) unclassified (also called “unexplained” anemia). These broad categories should not be seen as exclusive, as more than one type of anemia maybe present. For example, in patients with anemia of CKD, nutrient absolute or functional nutrient deficiency, and/or chronic inflammation may also contribute to impaired erythropoiesis and anemia. Other schemes for classification of anemia exist, including ones appropriately more refined for the investigation of causes of anemia in the clinical setting (see below).
Anemia with nutrient deficiency is common. In a recent study that analyzed NHANES III data from community-dwelling adults 65 years and older, approximately one third of all causes of anemia met the study criteria for iron, folate, and/or vitamin B-12 deficiency.
Iron deficiency is by far the most common cause of nutrient-deficient anemia. Along with protoporphyrin IX, iron is a key component of the heme complex, the nonprotein pigment of the hemoglobin molecule in RBCs that is directly involved with oxygen binding and transport. Reduced availability of iron may cause anemia through impaired Hb synthesis. Iron-deficiency anemia is usually the endpoint of a negative overall iron balance disorder that gradually progresses over time. While decreased oral iron intake as well as reduced absorption maybe important contributing factors, it is believed that iron-deficiency anemia in older adults is primarily caused by chronic blood loss, in particular from the gastrointestinal (GI) tract. Iron-deficiency anemia constitutes an advanced stage of iron deficiency. The earlier stages of iron deficiency are marked by progressive depletion of iron stores. With exhaustion of iron stores, Hb synthesis becomes compromised, and results in erythropoiesis impairment. Eventually, Hb drops below specific thresholds, and anemia is diagnosed. Early in iron-deficiency anemia, anemia maybe normochromic and normocytic. Increased variation in RBC size may be observed, as assessed by an increase in red cell distribution width (RDW) in automated analysis of RBCs. As the duration and severity of iron deficiency increases, overt iron-deficiency anemia ensues. At this more advanced stage, the production of microcytic (small-sized), hypochromic (with decreased Hb content) erythrocytes is characteristic. Other typical laboratory abnormalities of advanced, overt iron-deficiency anemia include low serum ferritin concentration, low serum iron concentration, increased total iron-binding capacity (TIBC), low transferrin saturation ratio (serum iron divided by TIBC), and increased levels of soluble transferrin receptor (sTfR) (see below). Complementary laboratory tests maybe useful to further characterize the different stages of iron deficiency and iron-deficiency anemia.
The remaining cases of nutrient-deficiency anemia are largely caused by deficiency of folate and/or vitamin B-12. These deficiencies may impair erythrocyte maturation and proliferation through their adverse effect on DNA synthesis and result in macrocytic anemia, i.e., anemia with oversized RBCs, usually quantified as a mean corpuscular volume (MCV) greater than 100 femtoliters (fL). Analogously to iron deficiency, vitamin B-12 deficiency usually progresses across stages of severity. In its earlier stages, one is able to maintain erythropoiesis using vitamin B-12 from body stores. After, stores become depleted, erythropoiesis becomes impaired, and megalobastic anemia ensues. Often, vitamin B-12 deficiency, as assessed by low serum vitamin B-12 levels, as well as macrocytic changes maybe noted before the drop of Hb below the level in which anemia is diagnosed. Typically, other laboratory abnormalities that accompany the macrocytic anemia of vitamin B-12 deficiency include the presence of hypersegmented granulocytes on blood smears; giant platelets may also be observed. Thrombocytopenia, as well as leucopenia may also occur. Presence of iron deficiency concomitant to vitamin B-12 deficiency anemia may mask typical abnormalities of the latter. Often, vitamin B-12 deficiency anemia is caused by gastric and intestinal disorders that prevent the proper digestion of vitamin B-12, involving either impaired release of cobalamin from the ingested food and/or the absorption of free cobalamin in the GI tract. Examples of these disorders include hypochlorhydria, surgical procedures involving the stomach, atrophic gastritis (involving a deficiency in the production of intrinsic factor by parietal cells of the gastric mucosa), pancreatic insufficiency, and small bowel bacterial overgrowth. Vitamin B-12 deficiency, resulting from dietary deficiency is not common, but may occur in subjects with severe long-term avoidance to meat products, as maybe found in strict vegetarians. Folate deficiency, on the other hand, is often caused by poor nutritional intake. GI disorders result in decreased absorption of folate (e.g., hypochlorhydria associated with atrophic gastritis or acid-suppressive therapy, celiac disease) and adverse effects of drugs (e.g., alcohol, methotrexate, anticonvulsants). Like vitamin B-12 deficiency, folate deficiency typically presents as a macrocytic anemia. Laboratory tests are useful to distinguish folate- versus vitamin B-12 deficiency. The distinction is important vis-à-vis therapeutic implications (see below). Other vitamins and trace minerals have also been hypothesized to impair erythropoiesis, and potentially contribute to the development of anemia, when other mechanisms are also present, including deficiencies in vitamins A, C, E, and pyridoxine and riboflavin (vitamin B group), as well as copper, selenium, and zinc.
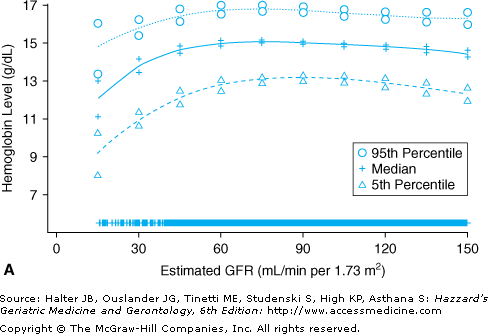
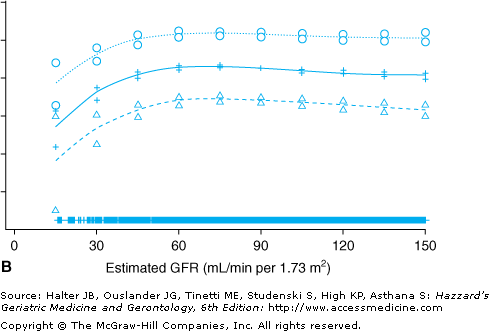
With increasing age, the prevalence of CKD increases, kidney function declines, and the prevalence of anemia—a common complication of CKD, particularly when the latter is advanced—increases. Figure 102-6 displays data on the age-adjusted, cross-sectional relationship between renal function, as assessed by estimated glomerular filtration rate (eGFR) levels, and the continuous distribution of Hb observed in a nationally representative sample of 15 625 noninstitutionalized Americans 20 years and older. Stable Hb levels were observed across eGFR levels greater than 60 mL/min per 1.73 m2; below this threshold, lower eGFR levels were associated with progressively lower Hb levels. In another study that analyzed NHANES III data and used a strict definition for anemia of CKD, 8.2% of all cases of anemia were attributed to anemia of CKD only, and an additional 4.3% to anemia of CKD in combination with ACI (see below). The definition of anemia of CKD in that study was the following: Hb <12 g/dL in women and <13 g/dL in men, an eGFR less than 30 mL/min per 1.73 m2, and no evidence of iron, folate, or vitamin B-12 deficiency. Had more sensitive eGFR cutoffs (e.g., eGFR <45 or <60 mL/min per 1.73 m2) been used to define anemia of CKD, higher prevalence estimates of this type of anemia would have been yielded. Typically, anemia of CKD is normocytic and normochromic. The primary mechanism through which CKD causes anemia is a deficiency in the production of erythropoietin, a naturally occurring erythropoiesis-stimulating hormone mainly produced by the kidneys in adults, which is secreted in response to hypoxia. The diminished renal production of erythropoietin is most noticeable in the most advanced stages of renal failure. In the failing kidney, in addition to the absolute deficiency in erythropoietin, the decline in the renal excretory function also plays a part in the pathogenesis of anemia, as it may contribute to a shortened life span of RBCs and bone marrow suppression. Other concomitant factors may also inhibit erythropoiesis in anemic subjects with CKD, including nutrient deficiency, subsequent to blood loss, decreased oral intake, and dialysis removal, as well as increased iron utilization during therapy with erythropoiesis stimulating agents (see below). Increasing attention has been paid to the role of inflammation as a mechanistic factor in the pathogenesis of anemia in CKD patients (see below).
Figure 102-6.
Median and 5th and 95th percentiles of hemoglobin levels among men (A) and women (B) 20 yrs and older who participated in the Third National Health and Nutrition Examination Survey (1988–1994). All values are adjusted to the age of 60 yrs. Estimates and 95% confidence intervals are demarcated at selected levels of estimated glomerular filtration rate (GFR). Marks near the abscissa indicate the estimated GFRs of individual data points. (Astor BC, Muntner P, Levin A, et al. Association of kidney function with anemia: the Third National Health and Nutrition Examination Survey (1988–1994). Arch Intern Med. 2002;162(12):1401–1408, reprinted with permission.)
Traditionally, this type of anemia has been referred to as anemia of chronic disease. Recently, to more properly capture the inflammatory nature of this condition, the term anemia of chronic inflammation (ACI) started to be used. In a recent study that analyzed data from a large population-based sample of community-dwelling adults 65 years and older (NHANES III), more than 20% of all cases of anemia met the criteria for ACI. In that study, the overall prevalence of ACI (24%) was slightly higher than that attributed to anemia with iron deficiency (20%). ACI is associated with a wide variety of inflammatory conditions, including chronic infection, cancer, diabetes, rheumatoid arthritis, and congestive heart failure, and trauma. It is said that the severity of ACI and that of the underlying disease(s) are positively correlated.
Several mechanisms mediated by inflammatory cytokines have been proposed to explain ACI. The key one involves unavailability of iron for erythropoiesis despite adequate, if not elevated total iron stores, mediated by inflammation. This dysregulation of iron homeostasis results from sequestration of iron in the cells of the reticuloendothelial system, with consequent inadequate availability of iron for erythropoiesis. Interleukin-6 (IL-6), a proinflammatory cytokine, has been shown to stimulate the hepatic production of hepcidin, a polypeptide that is a major regulator of iron homeostasis. Hepcidin inhibits the release of iron from the reticuloendothelial and macrophage systems, as well as iron intestinal absorption. High levels of IL-6 have been shown to induce an increased production of hepcidin, which in turn can lead to sequestration of iron by reticuloendothelial cells, reduced amount of iron available for erythropoiesis, and, ultimately anemia. Additional mechanisms maybe involved in the pathogenesis of ACI, including the inhibition of the proliferation and differentiation of erythroid progenitor cells, which may result from inflammation-mediated induction of apoptosis of progenitor cells, and/or through a direct toxic effect of inflammation on progenitor cells. Another mechanism involves a blunted erythropoietin response, i.e., an attenuated compensatory response in erythropoietin production in relation to the degree of anemia, resulting in a relative erythropoietin deficiency. Support for this explanation include in vitro evidence of inhibition of erythropoietin production by proinflammatory cytokines, such as tumor necrosis factor alpha (TNF-α) and interleukin-1, as well as evidence of lower levels of serum erythropoietin in patients with ACI as compared to patients with iron-deficiency anemia for the same level of anemia. Controversy exists though as to whether the iron-deficiency group is a proper control group, given its exacerbating impact on hypoxia homeostatic mechanisms. Shortened RBC life span may also contribute to the pathogenesis of ACI.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree