Study
No. of patients
Age at ITP diagnosis (year)
Duration before rituximab (month)
Age at rituximab treatment (year)
Times of injection
Duration of observation (month)
Rate of initial response (CR + PR)
Rate of complete response
Relapsed patients after initial response (%)
Duration until relapse
Patients with severe infection
Patients with serum sickness
Reference
1
24
24 (6–120)
NA
4
9
19 (79)
15 (63)
8 (42)
7.5 (3–18)
0
3
[10]
2
22
5.8 (2.5–15.2)
44 (2–27)
NA
1
NA
13 (59)
7 (47)
5 (38)
9 (4–24)
0
0
[11]
4
36
NA
NA (7–145)
11.2 (2.6–18.3)
4
>12
11 (30)
NA
3 (27)
9 (8–11)
0
2
3
19
NA (3–18)
NA (1–48)
NA
4–6
18
15 (79)
10 (67)
1 (7)
NA
0
0
[14]
5
49
7.4 (0.7–17.6)
21 (1–175)
10.7 (1.2–17.7)
4–6
39.5
34 (69)
26 (53)
13 (38)
4 (1–8)
0
0
[15]
6
22
4.2 (0.3–11.6)
18.5 (2–120)
5 (0.5–20)
1–4
76.8 (62.4–85.2)
11 (50)
9 (41)
8 (72)
(2–26)
0
1
[16]
As shown in Fig. 1, the downward tendency of relapse-free survival (RFS) after initial response is one of major concerns to therapeutic effectiveness of rituximab [15, 16]. From a long-term follow-up conducted for more than 5 years, Patel et al. showed that 52% (34/66) of initial responders subsequently relapsed. Actually, 28 relapsed within 1 year and 6 relapsed during 1–2 years, but none relapsed after 2 years, suggesting that observation for at least 2 years is necessary to assess the long-term efficacy of rituximab for children with chronic ITP [17].
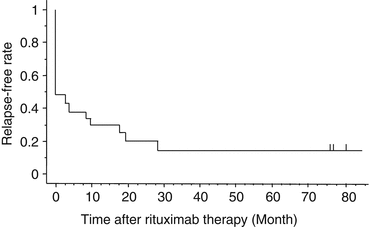

Fig. 1
Relapse-free survival rate of subjects who keep continuing first response to the first course of rituximab during long-term follow-up. The gray-shaded area of the initial 4 weeks indicates the evaluation period of the initial response to rituximab [16]
A recent meta-analysis including 324 pediatric patients showed that a pooled CR (platelet count >100 × 109/L) rate and an overall response (platelet count >30 × 109/L) rate were 39 and 68%, respectively [18]. Rituximab therapy might be promising for refractory ITP patients, and, therefore, it might offer relief from bleeding symptoms and allow for avoidance of splenectomy. However, infection is a major concern, and there have been reports in children of pneumonia, varicella, and reactivation of hepatitis C [4, 18–20].
The relationship of clinical variables with response of rituximab and relapse-free factors has been studied [15, 17, 21]. Patel et al. [17] reported that patients showing a higher degree of response continued to remain in remission for a long-term period compared to those with a lesser degree of response (relapse rate within 1 year; 7% (2/28) in CR vs. 40% (4/10) in PR). Nor were significant predictors of splenectomy, gender, or age for response of rituximab and relapse-free factors. Although the efficacy of rituximab was not high, Matsubara et al. indicated that the initial responders to rituximab achieved remission at significantly higher rate, even after relapse than nonresponders did. Consequently, in clinical practice, the initial response is a useful indicator of the subsequent remission rate.
Moreover, the rituximab treatment regimen is not necessarily consistent among ITP patients. A recent systematic review pointed that there is no “standard” dose for rituximab treatment in children [18].
4.1 Development of TPO-R Agents
For refractory or chronic children with ITP whose risk of severe bleeding would grow even with the second-line treatments, a strong desire has persisted for therapeutic agents with distinct action mechanisms. The discovery and development of a human recombinant TPO (rh-TPO, the first generation of TPO) proved that the activation of TPO receptor can increase thrombopoiesis of megakaryocytes, facilitating TPO-based therapies as treatment for ITP [22–24].
However, the initial clinical trials demonstrated that healthy volunteers who received rh-TPO became severely thrombocytopenic because of cross-reactivity between autoantibodies to rh-TPO and endogenous TPO. This result led to the development of new agents that stimulate TPO receptor but led to little immunogenic adverse effect. The newly developed TPO-R agonists (the second generation of TPO) belong to either TPO non-peptide mimetics (eltrombopag) or TPO peptide mimetics (romiplostim) that increase platelet production by promoting the maturation of BM megakaryocytes through activation of TPO receptor signaling.
The properties of eltrombopag (daily p.o. medicine) and romiplostim (weekly SC injection) are presented in Table 2. The approved administration of eltrombopag is 50 mg/day initial dose and 75 mg/day maximum in Europe and the United States, but for the East Asian patients, the applied dosage was reduced to 12.5 mg/day initial and 50 mg/day maximum because of ethnic difference of drug responsiveness [25].
Table 2
Comparative study of hemorrhagic manifestations between pediatric and adult patients with ITP
Eltrombopag | Romiplostim | |
|---|---|---|
Compound | • A low-molecular compound activating TPO receptor | • An Fc peptide fusion peptibody binding to TPO receptor |
Indication | • Adult ITP to which pretreatment is ineffective | • Adult ITP to which pretreatment is ineffective |
Dose and effects | • Initial dose: 50 mg/daya • Maximum dose: 75 mg/day • Oral administration (daily) • PLT > 50 × 103/μL (6 week): 60–81% • Thrombocytopenia after discontinuation • Reduction of combined drugs | • Initial dose: 1 mg/kg/week • Maximum dose: 10 mg/kg/week • SC administration (weekly) • PLT > 50 × 103/μL (6 week): 63–88% • Thrombocytopenia after discontinuation • Reduction of combined drugs |
Affecting factors | • No conclusion on children or long-term administration • Little effects of platelet counts, pretreatments, concurrent drug, or splenectomy | • No conclusion on children or long-term administration • Little effects of splenectomy |
Adverse effects | • Headache, nasopharyngitis, liver dysfunction • Myelofibrosis • Thrombosis | • Headache • Myelofibrosis • Thrombosis |
Antibody | • No induction of TPO-inhibiting antibody | • Possible neutralizing antibody, but no cross reactivity to TPO |
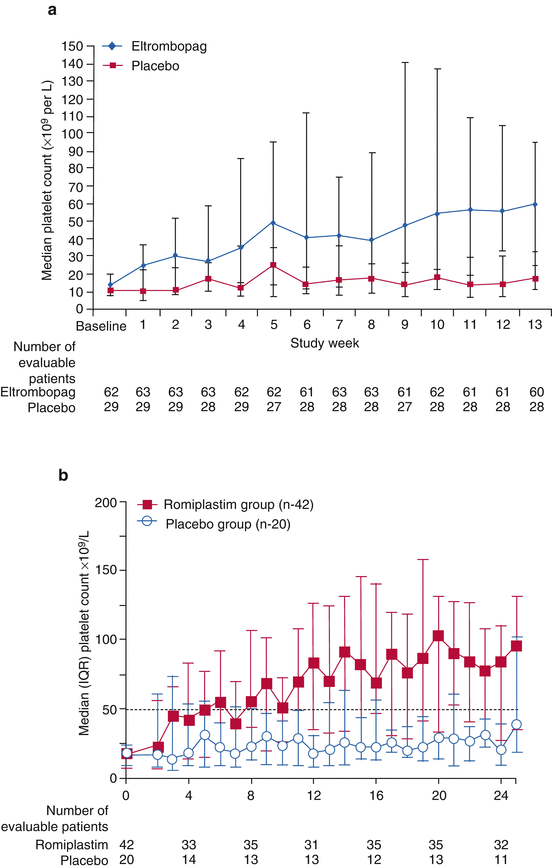
In the prospective and randomized studies for adult with chronic ITP, 109 of 135 patients (79%) showed significant increases of platelet counts, decreases of concurrent drugs, and lower demand of rescue therapies [26]. Therapeutic effects were not significantly influenced by prior treatments, previous splenectomy, or pretreatment platelet counts (Fig. 2).
For romiplostim, the initial dose is 5 μg/kg/week SC and adjusted up to 10 μg/kg/week at maximum with no marked racial difference of drug responsiveness. Similarly to eltrombopag, the efficacy of romiplostim was approximately 80% for adult with chronic ITP. In a randomized and double-blinded clinical trial for 125 patients [27], the percentage of patients who attained reduction or discontinuation of concurrent drugs was 87% for the romiplostim cohort and 38% for the placebo cohort. Moreover, a randomized clinical trial for patients who were refractory ITP without splenectomy, the subsequent execution rate of splenectomy was 9% for the romiplostim cohort and 36% for the standard therapy cohort, suggesting the possible avoidance of splenectomy by incorporating TPO-R agonists to treatment for refractory ITP [28].
The most common adverse effects of TPO-R agonists were headache, although nasopharyngitis and liver dysfunction were also observed for eltrombopag [26, 27, 29–31]. Thrombosis was reported to have a low rate of incidence, but its causal relation remains unclear [28, 31]. As long-term adverse effects, major concerns are the development of myelofibrosis, depletion of hematopoietic stem cells, and induction of other bone marrow abnormalities including malignant diseases. Further investigation of those areas of concern is expected to lead to better understanding.
4.2 Clinical Studies of TPO-R Agonists for Children
Recently, many reports have described the efficacy and safety of TPO-R agonists for children with chronic ITP who had been treated unsuccessfully with the first-line treatments (Table 3) [32–38]. Patients included children (1–17 years old) with chronic/refractory ITP who received romiplostim or eltrombopag at doses adjusted to less than the maximum dose to maintain platelet counts at least >50 × 109/L. In 2011, the first randomized clinical trials with romiplostim were reported: short-term observation (12 weeks) for 18 and 22 patients for whom the respective pretreatment duration was 2.4 and 2.5 years [32, 33]. These studies showed that the efficacy to maintain >50 × 109/L of platelet counts without rescue medication was 83.5–88% for patients with romiplostim, as compared to 0% for those with placebo. Nevertheless, the small number of patients and the short-term duration of treatment of these studies might limit the generalization of their conclusions. More recently, a larger study of 62 children (romiplostim, 42; placebo, 20) during 24 weeks of treatment duration revealed that the efficacy of romiplostim was 52% for the romiplostim group as compared to 10% for the placebo group [38]. Another randomized double-blinded and subsequent open-labeled study with eltrombopag has demonstrated its efficacy as 40% for the eltrombopag group, compared to 3% for those of placebo [37]. The efficacy of eltrombopag increased further to 80% (70/87 patients) of efficacy during the following 24 weeks of the open-labeled period. These two recent studies confirmed that both romiplostim and eltrombopag TPO-R agonists are effective for chronic/refractory ITP in children. For these periods, no drug resistance or autoantibody against intrinsic TPO was detected.
Table 3




Clinical trials of TPO-R agonists for children with ITP
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree