Fig. 1.1
Milk line from the axilla to the groin
The mammary ridge proliferates as a solid bud between the fifth and seventh week of gestation (Fig. 1.2). The mammary bud grows downward into the dermis and starts branching to the secondary bud around the twelfth week. This downward growth and branching is due to inductive influence of the extracellular matrix of the primary mesoderm on the mammary bud. This epithelial and mesenchymal signaling is through paracrine and juxtacrine mechanisms. The mesoderm and underlying adipose tissue around the bud produce growth factors and hormones, which interact with receptors on the mammary bud ectodermal cells to proliferate and grow downward. These hormones and growth factors derive from lipids from adipose tissue. These buds elongate to form lactiferous ducts at about the twentieth week.
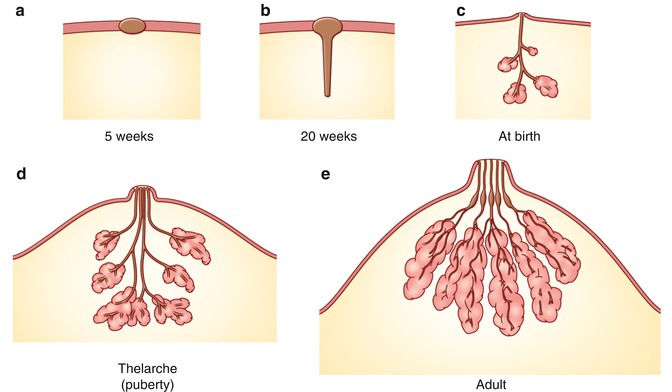

Fig. 1.2
Development of breast. (a) Mammary ridge. (b) Elongation of the mammary bud and canalization of the lactiferous duct in 20 weeks. (c) Rudimentary mammary glands. (d) Further development to form the adult ductal system in the mammary gland during puberty. Connective tissue and deposition of fat. (e) Functional gland with secretary alveoli
The canalization of the mammary bud that is transformed into lactiferous ducts is influenced by placental hormones that are circulating through the fetal circulation. The placental hormones consist of progesterone, growth hormone, insulin-like growth hormone, estrogen, prolactin, adrenoglucocorticoid, and triiodothyronine. There are about 15–20 lobes of glandular tissue formed with lactiferous ducts. The mammary gland is surrounded by mesenchyme, which forms connective tissue, fat, and vasculature and intersects mammary nerves.
By the end of prenatal life, the mammary ectoderm, with modified apocrine glands, branches into 15–20 solid buds that then canalize and form the lactiferous ducts and lobes of the lung alveoli. The mammary gland proliferates and the ducts elongate; further divisions occur to form the mammary glands and ductal system. Initially, the lactiferous ducts open into a small mammary epithelial pit, which is transformed into the nipple by proliferation of the underlying mesenchyme. Mesodermal proliferation also gives rise to the circular and longitudinal smooth muscle fibers of the nipple-areola complex. A failure of mesenchymal proliferation causes the ducts to open into a shallow pit resulting in an inverted nipple. At birth, the male and female anatomies appear alike due to maternal circulating prolactin in the mother. While the male breast remains the same, the female breast undergoes further transformation at the time of puberty, pregnancy, and lactation due to hormonal influence.
Areola
During the neonatal period, the nipple is a small pit at the center of the thickened areola. The areola contains sweat glands and sebaceous gland with its Montgomery tubercle. The nipples become elevated and protrude, with the areola developing increased pigmentation. Prolactin secretion in the pituitary gland is stimulated by falling estrogen levels in the neonatal period and may result in the acini to develop and produce witch milk, milk secreted from the breasts of newborns [6].
Congenital and Acquired Deformity of the Breast
Occasionally, fragments of the mammary ridge may persist, giving rise to accessory nipples (polythelia) or developing into a complete breast, also known as polymastia, along the mammary line (Fig. 1.3) [7–11]. The most common location of an accessory nipple is within the inframammary fold, while accessory breasts are most commonly found in the axilla (Figs. 1.4 and 1.5).
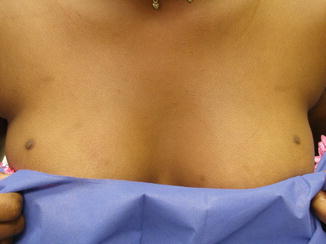
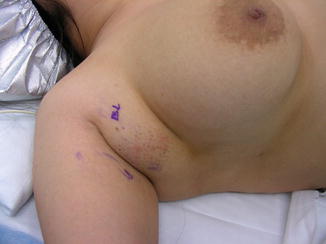


Fig. 1.3
Accessory nipple. Accessory nipple seen on the superior aspect of both breasts

Fig. 1.4
Axillary breast. Patient has axillary breast in the axilla of the right breast

Fig. 1.5
Axillary breast. The patient has bilateral axillary breasts
There is a wide variation of congenital and acquired deformities (Table 1.1). For example, the congenital deformities are hyperplasia, hypoplasia, and a combination of these. The hyperplastic breasts are unilateral or bilateral with hyperplasia, polythelia, polymastia, hematoma, and giant fibroadenoma occurring in the female and gynecomastia in the male (Fig. 1.6). The hypoplasia of the breast may occur in one or both breasts. Hypoplasia can be seen in tuberous breasts due to a fibrous cord and the absence of superficial fascia under the areola causing hypertrophy and a herniated areola (Figs. 1.7 and 1.8). There are various abnormalities of the hypoplastic breast and athelia of the breast, which can occasionally be seen in Poland syndrome (Fig. 1.9).
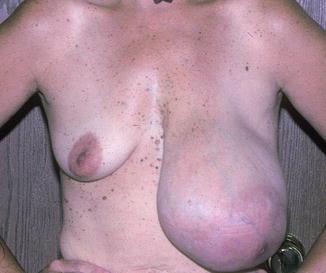


Table 1.1
Congenital and acquired deformity of the breast
Congenital | Acquired | ||
|---|---|---|---|
Hyperplastic breast | Hypoplasia of the breast | Iatrogenic | Trauma |
Hypertrophy | Hypoplasia | Thoracotomy | Thermal burn |
Unilateral and bilateral | Unilateral and bilateral | Breast biopsy | Penetrating injury to the breast |
Polythelia | Athelia | Lumpectomy | Breast ironing |
Polymastia | Poland syndrome | Radiation | |
Male gynecomastia | Tuberous breast | ||
Giant fibroadenoma | Inverted nipple | ||

Fig. 1.6
Hematoma of the breast. This patient presents with a hematoma of the left breast and hypoplasia of the right breast. It is slow in growth, 5 years

Fig. 1.7
Asymmetry breast. This patient has hypertrophy breast with asymmetry

Fig. 1.8
Asymmetrical breast. This patient has hypertrophy of the breast with asymmetry

Fig. 1.9
Poland syndrome. Patient has hypoplasia of the right breast, absence of pectoral muscle, and a small areola. The patient has nipple tattooing of the right breast. The patient has mastopexy with implant on the left side
The acquired deformity is due to iatrogenic injury caused by thoracotomy or biopsy of the breast for a tumor, and post-radiation deformity is due to radiation for a hemangioma or tumor. Such injuries may be the result of thermal burns in childhood that may cause scars, contracture, and deformity. Other examples describe an alarming number of worldwide reports that have identified vicious practices of “breast ironing” [12] in teens in order to suppress the growth of breasts and prevent teen pregnancy in Central and West Africa.
Development and Physiology of the Breast Parenchyma
During puberty, enlargement of the mammary glands is primarily due to ovarian estrogen. The lactiferous ducts branch to form a solid spherical mass of glandular cells that are potentially still able to develop into alveoli. At the end of puberty, there is dense fibrous stroma that separates the scattered ducts lined with epithelium and fat in the mammary gland. Increasing serum estradiol concentrations promote fat deposition and the formation of new ducts by branching and elongation.
During pregnancy, the lactiferous ducts branch and secretory alveoli appear due to the influence of placental progesterone, estrogen, prolactin, and lactogen. The adipose tissue increases under the influence of a robust blood flow.
The abrupt withdrawal of progesterone upon delivery leaves the breast under the influence of prolactin. In the presence of growth hormone, insulin, and cortisol, prolactin converts the epithelial cells to secretory cells, resulting in the production of milk by alveolar cells. Oxytocin is then released from the posterior pituitary gland in response to nipple-areola stimulation, causing the ductal myoepithelial cells to contract and eject milk. After parturition, there is reduction of estrogen and progesterone levels that stimulates prolactin secretion from the anterior hypophysis. This promotes the apocrine gland to secrete milk with fat droplets. Placental lactogen and sex hormones maintain the mammary epithelium in a pre-secretory phase by antagonizing the effects of prolactin and thyroid hormone [6]. During the post-lactational period, the prolactin level decreases with an inhibitory effect of non-expelled milk that results in a return to a nonfunctional state. During the pre- and postmenopausal period, the gland becomes senescent, with resulting involution of the mammary gland and deposition of connective tissue and fat [5].
Adult Breast
Overview
The breasts are located on the anterior chest wall and are composed of the skin envelope which contains the nipple-areola complex with adnexal structures and mammary parenchyma containing glandular tissue and adipose and fibrous connective tissue which support the gland (Fig. 1.10). The adult female breast rests within the superficial and deep fascia held in position with suspensory ligaments on the anterior upper thoracic wall. The breast extends from the second rib to the inframammary fold located at the level of the sixth or seventh rib [13–15]. Medial extension of the breast is to the sternum, and the lateral border extends to the mid-axillary line terminating as the axillary tail of Spence. The posterior surface of the gland rests upon the fascia of the pectoralis major and serratus anterior muscle. The inferior aspect rests on the external oblique muscle and upper portion of the rectus sheath with inframammary support.
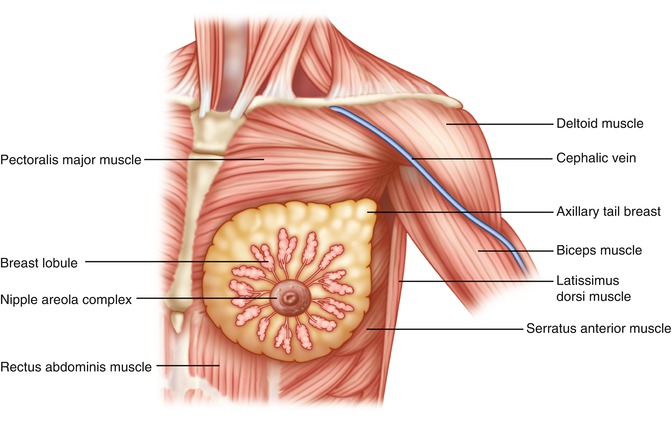

Fig. 1.10
Anterior view: breast shows mammary glands, lobules situated over the pectoralis major muscle, axillary tail projecting into the axilla
The size and shape of the breast vary with individual race and age. The breast can develop into a variety of shapes, including hemispherical, conical, pendulous, tuberous, piriform, thin, or flattened. Due to the development of the human infant jaw relative to primates, the conical shape of the breast is primarily due to fat accumulation during lactation period aids. This aids with breastfeeding without suffocation of the baby and further helps to feed without retraction of the pre-maxilla.
Skin Envelope and Nipple-Areola Complex
The skin of the breast surrounds the underlying parenchyma, with the nipple-areola complex located at the apex of the breast. The quality of the skin varies from patient to patient. During youth, the skin has greater elasticity and provides firm support to the underlying parenchyma. With age, weight gain, and pregnancy, the skin loses its elasticity, becoming thinner and often developing stria (tears and separations in the thinned dermis) with diminished support of the underlying parenchyma. Thinning of the central breast skin and absence of elastic support of the parenchyma can lead to a constricted breast associated with tubular or tuberous breasts.
The nipple stays within the center of the areolar skin which is of ectodermal origin. It contains sebaceous glands that when enlarged form Montgomery tubercles. The areola also has sweat glands that secrete lipoid material that helps lubricate and protect this structure. The areolar size ranges from about 28 to 50 mm in diameter for the typical breast. Enlarging during pregnancy, the areola is under hormonal influence that results in hyperpigmentation of the nipple and areola. The areolar pigmentation depends upon two polymers, eumelanin and pheomelanin, which cause brown and red pigments to be secreted. The extent of pigmentation varies with skin tone. The areola and nipple have no hair and are devoid of fat underneath the areolar skin. The areola is composed of sweat and sebaceous glands located within the periphery and the accessory glands of Montgomery, which produce small elevations on the surface of the areola.
The areola secretes both lubricants and pheromones to facilitate breastfeeding, with the latter facilitating the baby to nurse. It has been suggested that the differing color of the nipple-areola complex is for a higher visibility for the infant. The nipple contains openings for the lactiferous ducts and smooth muscle that aid in lactation and breastfeeding. The areola also contains tissues that cause erection of the nipple-areola complex during lactation.
The nipple stays at the level of the fourth intercostal space in the nulliparous woman or young adult. The nipple contains multiple sensory nerve endings, Meissner’s corpuscles, Ruffini’s corpuscles, Krause’s corpuscles, and autonomic nervous system. The sensory innervation of the nipple plays a great functional significance during lactation. The nipple contains openings for the lactiferous ducts and circular nonstriated smooth muscle that aid in lactation and breastfeeding. The longitudinal muscle may retract the nipple and circular muscles that, in turn, causes erection of the nipple.
Parenchyma of the Breast
The parenchyma of the breast is composed of the glandular tissue supported by fibrous tissue that holds the gland and interlobular adipose tissue that is also enriched with blood vessels and nerves. The breast parenchyma is pale yellow in color, with the lobulated tissue supported by connective tissue. It is usually composed of 15–20 lobes, with each lobe comprised of the same number of tubuloalveolar lobules connected by a single lactiferous duct. They are arranged in a radial pattern and orientation from the areola. These lobules drain to the lobes through the lactiferous duct and sinuses just underneath the areola. The sinuses are reservoirs that are connected to the narrow papilla that transmit milk to the orifices in the nipple. The lactiferous ducts, lined with stratified squamous epithelium, transition into lactiferous sinuses lined with cuboidal and myoepithelial cells located beneath the areola.
Each of the lobules contains hundreds of secretory acini. The ducts have columnar epithelia and lined by the basal lamina and myoepithelium at the periphery of the ducts. It is from within these ducts that invasive ductal carcinoma arises. Larger ducts have two or three layers of epithelium, becoming keratinizing stratified squamous epithelium at the opening. The morphology of the secreting gland varies greatly with age and hormonal influence. The inactive breast undergoes mild cyclical changes associated with the menstrual cycle. Conversely, significant cellular hypertrophy of the breast occurs throughout pregnancy. The breast also contains varying amounts of fat, which contributes to the contour, shape, and softness of the breast. Fat deposition is influenced by genetic and hormonal factors. For example, postmenopausal women have more fat in their breasts, often making it easier to evaluate the tissue with mammography [16]. Lacking the alveoli, the male breast tissue is structurally different than the female breast. The male breast ducts are solid with little adipose tissue and no extension of the ducts beyond the areola. The nipple papilla and areola are small.
Gynecomastia
The early stages of breast development are independent of the sex steroid hormones, causing similarity of the breasts in both genders. During this time, the male mammary glands become responsive to the hormonal environment, with the presence of testosterone leading to the normal involution of the male mammary gland. However, in testicular feminization syndrome, where there are higher circulating levels of testosterone and a lack of testosterone receptors, the individual develops a female phenotype, including the typical female breast development.
Fascia and Ligaments
Scarpa’s fascia extends onto the chest and splits into an anterior and posterior lamella that encompasses the breast (Fig. 1.11). The anterior lamella forms inframammary fold and encloses the breast [13, 14, 17, 18]. The anterior lamella serves as the dissection plane when performing a mastectomy. The posterior lamella [19] separates the breast from the underlying pectoralis major muscle and is the plane of dissection for sub-glandular breast augmentation. There is a retromammary space between the breast parenchyma and posterior lamella on the pectoralis major muscle. This is called the loose areolar membranous layer and allows the overlying breast tissue to move upon the pectoral fascia. Occasionally, we see invasion of the fascia and muscle with invasive breast cancer, with the capacity to also invade the suspensory ligaments and retromammary space. The latter is often associated with visible skin retraction and lymphatic tumor cell invasion of the skin, referred to as peau d’orange, for its typical thickened appearance of the skin similar to that of an orange peel.
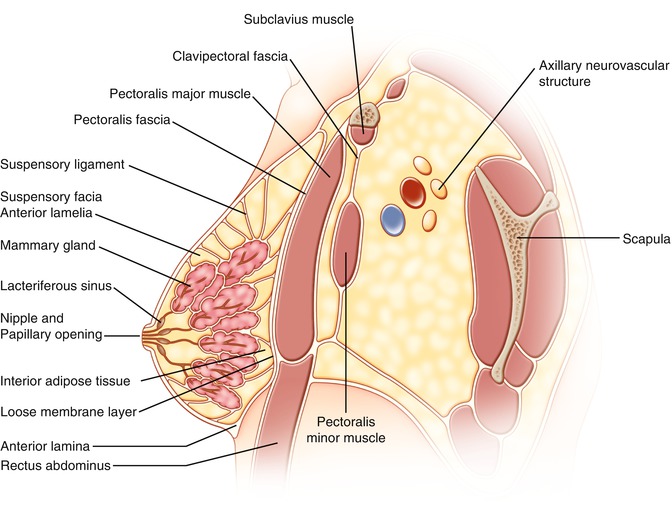

Fig. 1.11
Lateral view of breast. The rectus fascia, Scarpa’s fascia, splits into the anterior and posterior laminae. The posterior lamina called pectoral fascia spreads across the pectoralis major muscle and to the clavicle. The anterior lamina (fascia) envelops the breast. The suspensory ligaments anchor the anterior lamina and mammary gland to the pectoral fascia. The clavipectoral fascia attached to the clavicle splits around subclavius muscle and to the pectoralis minor and reaches the axilla through which the axillary vessels and nerves travel to the axilla
There are multiple interdigitating connective tissue fibers within the breast and between the lamellae called Cooper’s ligaments, which contribute to the shape of the breast and support it. In addition, there is a horizontal fascial septum originating from the lower, lateral border of the pectoral fascia at the level of the fifth rib that splits the breast into a superior two-thirds and inferior one-third. This septum was first described by Wuringer and colleagues and is associated with a well-formed neurovascular supply that courses within this fascia and provides support for the nipple-areola complex. The axillary tail of the breast has additional support via the suspensory ligament of the axilla. Retaining ligaments of the lateral chest wall suspend the lateral portion of the breast parenchyma and are often divided during a mastectomy [20].
Anatomy of the Axilla
The axilla is a distinct, pyramidal-shaped compartment located between the thoracic wall and the upper extremity and has four boundaries and one apex. The apex of the axilla extends into the posterior triangle of the neck via the cervicoaxillary canal. The anterior wall includes the pectoralis major and minor muscles as well as the clavipectoral fascia. The posterior wall is composed of the subscapularis, teres major, and latissimus dorsi muscles and overlying fascia. The medial wall is based on the serratus anterior muscle and the first four ribs that are covered by the intercostal muscles and fascia that is continuous with Scarp’s fascia. The lateral wall is bounded by the intertubercular sulcus of the medial humerus with the insertion of the latissimus dorsi, coracobrachialis, and biceps muscle. The base is composed of the axillary fascia that extends from the pectoralis major to the latissimus dorsi muscle and encloses the axillary contents.
The pectoral fascia invests the pectoralis major muscle, while the clavipectoral fascia extends from the clavicle to the axillary fascia within the floor of the axilla and encloses the pectoralis minor muscle. The part of the clavipectoral fascia attaches to the clavicle, enclosing the subclavius muscle to the first rib and referred to as Halsted’s ligament. It then unites to form one layer that covers the axillary neurovascular bundle. This fascia again divides to enclose the pectoralis minor muscle. This part of the fascia covers the axillary neurovascular bundle and axillary lymph nodes.
The fascia units below the pectoralis minor form the axillary fascia and terminate within the axilla as the suspensory ligaments of the axilla or Gerdy’s ligament (coracoaxillary fascia). This fascia is pierced by the cephalic vein, lateral pectoral nerve, and vascular branches from the thoracoacromial trunk. The suspensory ligament is pierced by the axillary tail of the breast (tail of Spence). The upper part of the clavipectoral fascia and Halsted’s ligament extends from the clavicle to the first rib, covering the thoracic inlet as an axillary sheath. The thickened fascia from the first rib to coracoid is called the costocoracoid ligament. A tubular sheath emerges from the thoracic inlet in order to cover the axillary neurovascular bundle, referred to as the axillary sheath. The axillary contents are composed of fibroadipose tissue that is pierced by the axillary tail, lymphatic system, and second lateral intercostal sensory nerves that innervate the upper inner aspect of the arm. The axilla also contains the great vessels and nerves that supply the upper extremity. These nerves and vessels are enclosed within the axillary sheath.
There are several nerves in the axilla that need to be identified during a formal axillary dissection. The long thoracic nerve, which is located on the medial wall of the axilla, arises from the C5 to C7 and enters the axilla via the cervicoaxillary canal. It lies along the lateral aspect of the serratus anterior muscle that it innervates. Injury to this nerve will result in the “winged scapula.” The thoracodorsal nerve originates from the posterior cord (C6 to C8) of the brachial plexus, accompanied by the subscapular artery and innervates the latissimus dorsi muscle. The intercostobrachial nerve is a purely sensory nerve that provides sensation to the skin of the axilla and the upper medial aspect of the arm.
Vascular Anatomy of the Breast
The breast receives blood supply from the perforating branches of the internal mammary artery medially, the lateral branches of the posterior intercostal arteries, and the lateral thoracic and pectoral branches of the thoracoacromial artery superiorly [21–25]. There is a mesentery as described by Wuringer that connects the lateral thoracic artery and chain of lateral intercostal vessels from the lateral border of pectoralis major to the medial anterior perforating intercostal vessels arising from the internal mammary artery.
These form the mesentery arc at the base of breast (Figs. 1.12 and 1.13) and traverse through the gland to the superficial vascular system (Fig. 1.14) [26–28]. The blood supply to the skin is primarily from the subdermal plexus with communications to the underlying perforators from the external mammary artery, anterolateral and anteromedial intercostal artery, and internal mammary artery. There is an abundant and substantial collateralization of arterial flow within the breast.
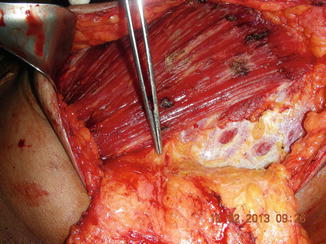
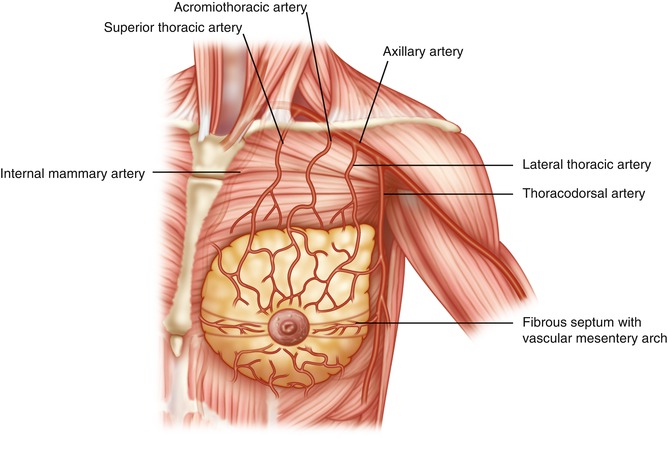

Fig. 1.12
Venous outflow. The subdermal plexus of the vein travels in a radial direction from the nipple-areola complex

Fig. 1.13
Vascular mesentery. This dissection shows lateral thoracic vessel arches, lateral to the pectoral muscle and form arc to supply blood flow to the breast

Fig. 1.14
Arterial vessels to the breast fibrous septum forming central pedicle to supply major part of the breast and nipple-areola complex described by Dr. E. Wuringer
The venous drainage of the breast closely follows the arterial blood supply and is directed towards the axilla. The superficial veins have extensive collateralization that may be visible just underneath the skin. The major venous drainage of the breast occurs through (A) perforating branches of the internal mammary artery, (B) tributaries of the axillary vein, and (C) perforating branches of the posterior intercostal veins. The posterior intercostal veins are in continuity with the vertebral plexus of veins, the Batson’s plexus that extends from the base of the skull to the sacrum. This pathway may provide a direct and efficient route of hematogenous spread of breast cancer to the skull, vertebrae, pelvic bones, and central nervous system [29]. The blood supply to the nipple-areola complex is contributed from both the parenchyma beneath and the rich subdermal plexus surrounding the complex [20, 23, 30–32].
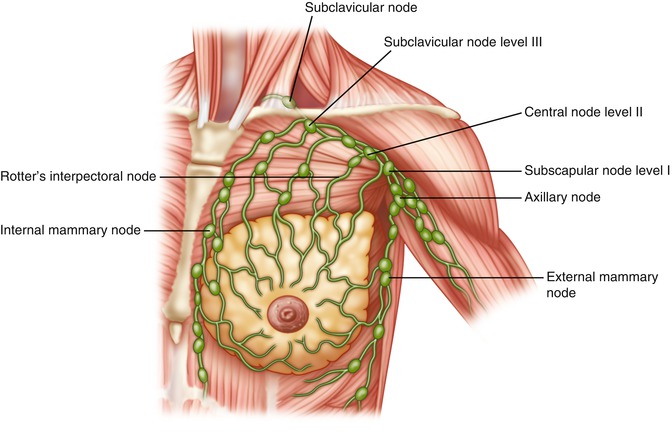
Lymphatic Drainage of the Breast
The lymphatics of the breast are located within the parenchyma itself, with individual lymphatic drainage to each of the lobules and ducts (Fig. 1.15). The main route of drainage of the breast, greater than 75 %, is through the axillary lymph node groups. The remainder of lymphatic drainage is through the parasternal nodes and likely the internal thoracic lymph nodes, which are a group of smaller lymph nodes located about a centimeter lateral to the sternal border. These nodes are located within the intercostal spaces along the internal mammary vessels. The skin of the breast drains via the superficial lymphatic vessels into the axillary lymph nodes.