5 Anatomic Imaging
Imaging is essential for the care of patients with cranial and spinal tumors. Imaging is used to detect and characterize lesions, establish a differential diagnosis, guide invasive diagnostic tests and therapy, and monitor change over time. This chapter provides an introduction to imaging the neuro-oncology patient, and discusses a general imaging approach, technique, tumor characterization, mass effect, and follow-up imaging.
 Imaging Approach
Imaging Approach
Computed tomography (CT) and magnetic resonance imaging (MRI) are the main imaging modalities for cranial and spinal neoplastic disease. When a patient presents with acute neurologic symptoms and a suspected or known cranial tumor, it is best to begin with a CT scan of the brain, as this is the quickest way to exclude conditions such as intracranial hemorrhage, brain herniation, and acute hydrocephalus, which may require urgent neurosurgical treatment. Computed tomography is widely available, and current multidetector scanners cover the whole head in about 30 seconds. Whether the CT is normal or abnormal, if a brain tumor is suspected, contrast-enhanced MRI is usually warranted for further evaluation. If symptom progression is subacute (over weeks to months) and MRI is available within a few days, it is reasonable to begin with MRI.
When a patient presents with symptoms of acute spinal cord or cauda equina compression (in the absence of trauma), imaging should begin with MRI, which provides excellent contrast among the spinal cord, cerebrospinal fluid (CSF), and other tissues in the spinal canal. If MRI is not possible, then the study of choice is CT myelography. This technique involves lumbar, or rarely C1-C2 cisternal, puncture and injection of iodinated contrast medium into the thecal sac under fluoroscopic guidance, followed by CT scanning through the spinal levels of interest. The contrast medium distributes in the CSF and delineates the subarachnoid space.
Pitfall
• Not all iodinated contrast agents are safe for intrathecal use; the agent must be chosen carefully.
 Technique
Technique
Computed tomography images are obtained by transmitting precisely collimated beams of X-rays through the body at multiple angles. Some X-rays are absorbed or scattered, and the remainder are transmitted through the patient to a detector opposite the X-ray source. From these data on X-ray transmission along each path, a computer algorithm derives the X-ray attenuation attributable to each location within the region imaged. These attenuation values, measured in Houns-field units, are then displayed as two-dimensional images. Advantages of CT compared with MRI are lower cost, wider availability, better access to critically ill patients during the examination, and greater tolerance of patient motion. Disadvantages include exposure to ionizing radiation and less contrast between soft tissues.
Whereas CT relies on X-ray transmission to create contrast between tissues, MRI uses electromagnetic waves in the radiofrequency range, and relies on a more complex interaction between this incident energy and tissue to generate images. The MRI scanner has a very strong and constant main magnetic field and additional milder time-varying magnetic fields. The latter magnetic fields are used to transmit electromagnetic waves into the body and then spatially localize the electromagnetic signals that are returned from the body. Advantages of MRI compared with CT are superior contrast between tissues1; availability of many different kinds of contrast between tissues; flexibility of adding a variety of advanced imaging techniques, such as spectroscopy, perfusion, diffusion tensor, and functional mapping of the cortex in the same examination as routine imaging; and lack of ionizing radiation.
Magnetic Resonance Imaging: Pulse Sequences
After a radiofrequency wave emitted by the scanner “excites” nuclei in the body, the body returns a signal to the MRI receiver. The characteristics of this returned signal depend on the local physical, chemical, and biological environment of the nuclei from which the signal arises; thus, the MRI signal reflects tissue characteristics. In particular, the waveform of the signal returned to the MRI receiver depends on how quickly the excited nuclei “relax” back to their initial state after radiofrequency excitation. There are two main types of relaxation: longitudinal and transverse. The time it takes for longitudinal magnetization to return to 63% of its equilibrium value after excitation is called “T1.” The time it takes for transverse magnetization to return 63% of the way to its equilibrium value (of zero) after excitation is called “T2.”
Different patterns (“pulse sequences”) of incident radiofrequency waves yield images with different types of contrast between tissues. A pulse sequence that generates images with tissue contrast reflecting mainly differences in T1 is called “T1-weighted,” and a sequence reflecting mainly differences in T2 is called “T2-weighted.” T2-weighted pulse sequences correct for inhomogeneities in the magnetic field. If this correction is removed, the resulting images are called “T2*-weighted” (pronounced “T2 star”) and are particularly sensitive to blood and calcification. Unfortunately, T2*-weighted images are also very sensitive to the magnetic inhomogeneities at interfaces between tissue and air, so the images are often distorted near the air-containing paranasal sinuses and temporal bones. T2*-weighted images are generated using a “gradient echo” type of pulse sequence. So-called susceptibility-weighted imaging is a newer variant of this sequence.
T2-weighted fluid-attenuated inversion recovery (FLAIR) is a type of T2-weighted pulse sequence that suppresses (i.e., turns black) signal from CSF. This sequence is helpful for detection of lesions near the margins of the ventricular system and at the surface of the brain. It is technically challenging to obtain complete suppression of CSF signal, especially in the posterior fossa, resulting in artifacts on FLAIR images. A diffusion-weighted pulse sequence yields images that reflect the degree of thermal or brownian motion of water molecules in tissue. Highly cellular tumors demonstrate restricted diffusion, as do some tumor mimics such as abscess. A diffusionweighted sequence generates diffusion-weighted images, which have a combination of T2 weighting and diffusion weighting, and an “apparent diffusion coefficient” (ADC) map that is only diffusion weighted. It is important to review both kinds of information to determine whether restricted diffusion is truly present. Steady-state pulse sequences are a group of sequences often used to obtain high spatial resolution images with very strong T2 weighting. They are ideal for examining cranial nerve lesions such as vestibular schwannomas, but offer poor tissue contrast within soft tissues such as the brain.
Each sequence has specific uses as well as advantages and disadvantages relative to the others. The MRI vendors have created unique names for their proprietary versions of these sequences, resulting in a confusing plethora of sequence names. A routine MRI protocol for brain tumor imaging includes a T1-weighted sequence before intravenous injection of contrast medium, an axial T2-weighted or T2-weighted FLAIR sequence, an axial diffusion-weighted sequence, an axial T2*-weighted sequence, and contrast-enhanced T1-weighted sequences.
Vascular Imaging
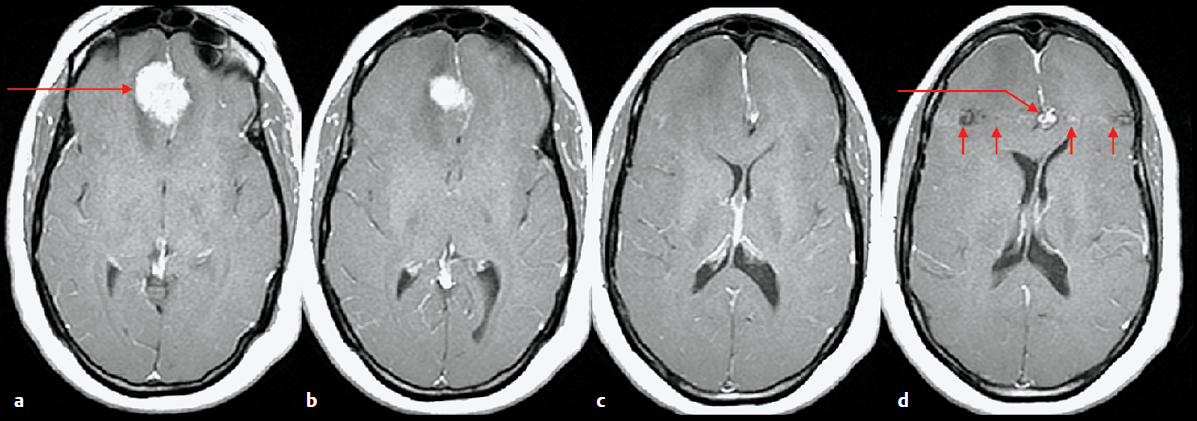
Vascular abnormalities are often apparent on images obtained using routine pulse sequences, and such findings can be important (Fig. 5.1). There are several methods for obtaining dedicated images of the arteries and veins. One option is the gadolinium-bolus magnetic resonance (MR) angiogram, but there are also options that do not require gadolinium such as time-of-flight MR angiography and phase-contrast MR angiography. The time-of-flight technique is based on nuclei from elsewhere in the body flowing into the slice that is being imaged, resulting in increased signal in vessels in that image. Computed tomography angiography is another technique for evaluating the arteries of the head and neck. It provides higher spatial resolution than MR angiography, but poorer contrast between arteries and background tissues. Catheter angiography is now rarely performed for diagnostic purposes in patients with tumors apart from occasional preoperative embolization of a highly vascular tumor. For preoperative planning, it is important to view not only postprocessed images, which provide a three-dimensional depiction of the blood vessels in isolation, but also the “source” images, which show the blood vessels in relation to the tumor.
Fig. 5.1a–d Evaluating vessels on routine MRI. (a-d) Axial contrast-enhanced T1-weighted images were obtained prior to resection of a presumed meningioma. Images demonstrate an extra-axial mass along the falx cerebri consistent with a meningioma (a, long arrow), but also a lesion along the course of the anterior cerebral arteries (d, long arrow) adjacent to the meningioma. The second lesion has “phase ghosting” artifact (d, small arrows) indicating that it is a vascular structure, and subsequent CT angiography confirmed a 9-mm aneurysm.
 Tumor Appearance
Tumor Appearance
Tumors generally have the appearance of a mass lesion; that is, they occupy space and displace normal structures. However, the mass effect of a small tumor may be difficult to appreciate, and primary brain tumors often infiltrate rather than grossly displace parenchyma, yielding less mass effect for their size than metastases or extra-axial tumors. Apart from lesion size and mass effect, the other main determinate of detectability is the CT attenuation or MR signal intensity contrast between a lesion and background tissue.2 Once a lesion is detected, the next step is characterization.
 Tumor Characterization
Tumor Characterization
Location
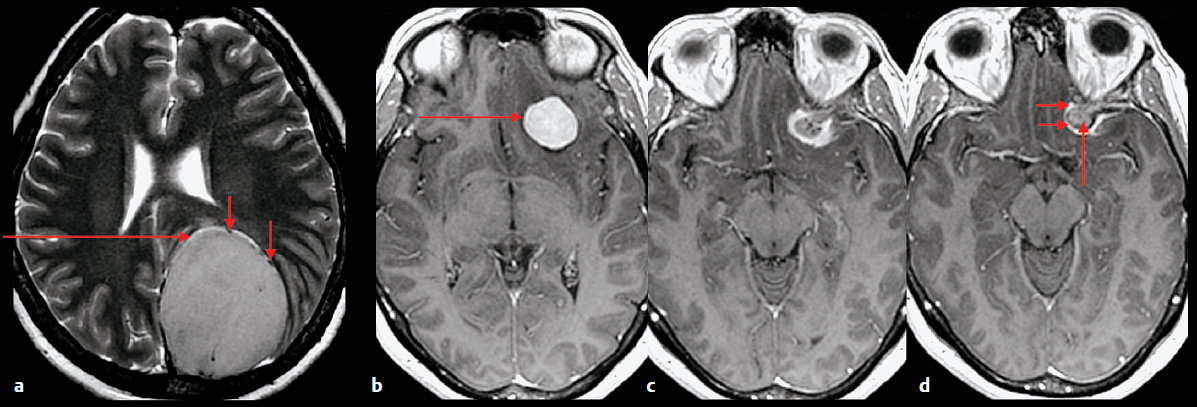
The first step is to determine whether a tumor arises from within the brain parenchyma (intra-axial) or from outside the brain (extra-axial). This initial distinction is a major determinant of the differential diagnosis, and to a degree, also portends prognosis. Several imaging findings suggest that a lesion is extra-axial: abnormality in the adjacent bone such as hyperostosis associated with a meningioma, a broad base of the lesion along the inner table of the calvaria or dura, associated dural enhancement, displacement of the brain away from the calvaria, dura between an epidural mass and the brain, widening of the subarachnoid space around the mass, a cleft of CSF or pial vessels coursing between the mass and the brain, and cortex between the mass and the white matter (Fig. 5.2).
Pearl
• Determining whether a tumor is intra-axial or extra-axial is the first step, and an extremely important step, in characterizing an intracranial tumor.
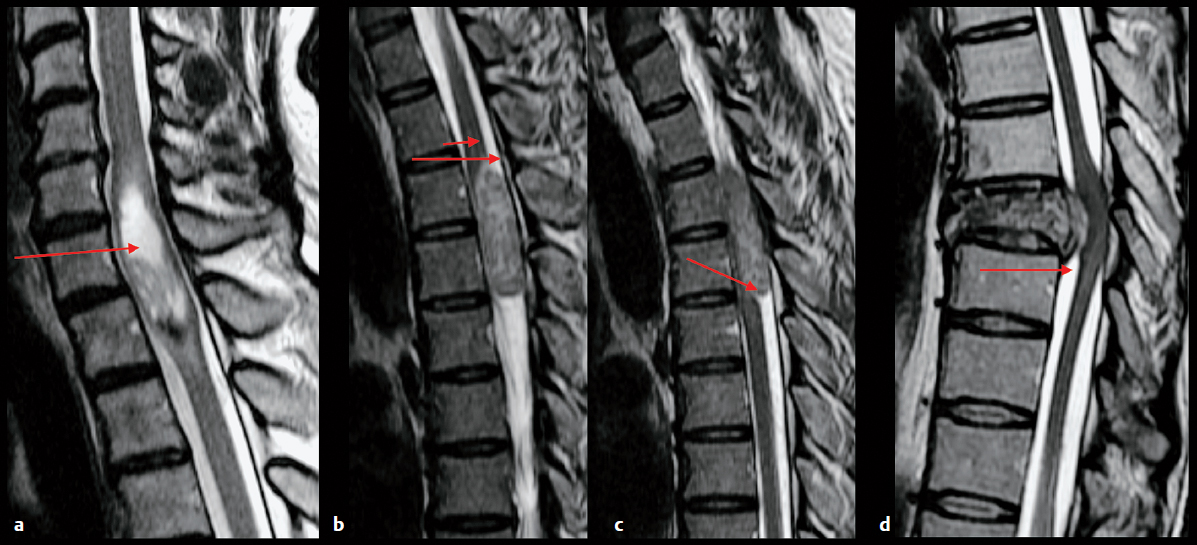
Once a tumor is categorized as intra-axial or extra-axial, more precise localization will help narrow the differential diagnosis, as many tumors tend to occur in specific locations. For example, oligodendroglioma typically arises at the junction between the cortex and subcortical white matter, whereas primary central nervous system (CNS) lymphoma typically arises in the periventricular region. Extra-axial lesions arise from specific structures such as the calvaria, dura, leptomeninges, vessels, and cranial nerves. For spinal lesions, it is helpful to categorize location as intramedullary, that is, arising from within the spinal cord, extramedullary intradural, or extradural (Fig. 5.3).
Multiplicity of tumors is a hallmark of metastatic disease, but metastatic disease can present as a single lesion, and many other neoplastic and nonneoplastic diseases can yield multiple lesions. For example, high-grade primary tumors such as glioblastoma can present with multiple sites of involvement, and patients with genetic disorders such as neurofibromatosis type 1 (gliomas and neurofibromas), neurofibromatosis type 2 (meningiomas, ependymomas, schwannomas), and von Hippel–Lindau syndrome (hemangioblastomas and endolymphatic sac tumors) commonly have multiple tumors.
Fig. 5.2a–d Features that indicate that a mass is extra-axial. (a) Axial T2-weighted image demonstrates a large mass in the left parietal region. There is a cleft of cerebrospinal fluid (long arrows) and there are vessels (short arrows) between the mass and the brain. (b–d) Contrast-enhanced axial T1-weighted images in a different patient shows an enhancing mass (long arrow in b) with adjacent dural enhancement (short arrows) and hyperostosis (long arrow in d) of the underlying bone of the anterior clinoid process.