Introduction
Amiodarone is a benzofuranic acid with potent antiarrhythmic properties that contains iodine amounting to 37% of its weight. Thus a 200-mg tablet (typical maintenance daily dose) provides approximately 70 mg of iodine of which 50% is bioavailable. This should be considered in the context of daily recommended iodine intake of 150 mcg per person. Consequently, amiodarone causes thyroid dysfunction in 15% to 20% of cases, with the incidence of thyrotoxicosis being approximately 3% to 9%. Amiodarone-induced hypothyroidism is more common in iodine-replete regions but fortunately easier to manage than the less commonly encountered entity of amiodarone-induced thyrotoxicosis (AIT). The approach to AIT has subtype, diagnostic, and management challenges, and at times this condition leads to life-threatening complications. This is due to multiple factors, including the use of this drug in patients with preexisting cardiovascular conditions, the lack of a consistently effective therapy, the prolonged half-life of amiodarone (2 to 3 months), and the fact that its discontinuation is not always feasible in the setting of life-threatening arrhythmias refractory to conventional antiarrhythmic medications.
AIT is particularly associated with increased mortality in older individuals and in those with impaired ventricular function, possibly due to a combination of preexisting cardiac dysfunction and a superimposed high-output thyrotoxic state, precipitating heart failure in these patients. Yiu et al. demonstrated that AIT predicted an adverse cardiovascular outcome in up to 31.6% and cardiovascular mortality in 12.6% of these patients. Similarly, O’Sullivan et al. demonstrated that AIT was associated with a mortality rate of 10% overall, but up to 50% in those with systolic heart failure (left ventricular ejection fraction less than 45%). These observations are consistent with the recent findings in France where AIT was shown to be the most common cause of thyroid storm in patients admitted to an intensive care unit (ICU). From a general viewpoint, any patient with AIT is at increased risk of deterioration because thyrotoxicosis can precipitate cardiac dysfunction even in asymptomatic patients, especially in those with cardiac conditions like systolic heart failure, ventricular arrhythmias, and congenital heart disease. The general approach to AIT and the role of specific therapeutic modalities in its management are discussed in the sections below.
Pathogenesis and Subtypes
Amiodarone causes thyroid dysfunction by one or a combination of the following mechanisms: (1) intrinsic drug factors that exhibit a dose-dependent direct toxic effect on thyroid follicular cells; and (2) iodine effects that include inhibition of deiodination of thyroxine (T4) to triiodothyronine (T3) but are further nuanced by the presence or absence of autoimmune thyroid disease or thyroid nodules. Classically, AIT has been categorized as type 1 or type 2, but many times the cases are mixed, especially at initial presentation, as shown in Table 2.1 . Type 1 AIT is characterized by iodine-induced hyperthyroidism due to excess hormone synthesis, usually occurring in patients with underlying thyroid disease such as nodular goiter or latent Graves’ disease. , , On the other hand, type 2 AIT is characterized by destructive thyroiditis causing excessive thyroid hormone release and is reported to occur in patients with normal thyroid or small goiter. , , Type 1 AIT has been more commonly reported in iodine-deficient areas, whereas type 2 AIT is more prevalent in iodine replete areas, , , , and is the most frequent form of AIT overall, accounting for 79% of cases in one large series. However, this clean separation is not evident in many cases that seem to have a mixed presentation with a combination of diagnostic features and probably mixed pathogenesis of both type 1 and type 2 AIT. , , ,
| Features of AIT | Type 1 AIT | Type 2 AIT | Mixed AIT |
|---|---|---|---|
| Mechanism | Excess thyroid hormone synthesis (iodine induced) | Excess release of thyroid hormone (destructive thyroiditis) | Features of both |
| Preexistent thyroid structure and function | Abnormal (nodules or latent Graves’ disease) | Apparently normal or small goiter | Features of both |
| Iodine-123 thyroid uptake | Most commonly low or low-normal, but sometimes normal or increased | Usually very low or absent | Features of both |
| Thyroid ultrasound with CFDS | Hypervascularity | Absent or decreased vascularity | Features of both |
| Initial treatment | Thionamides | Antiinflammatory therapy like prednisone | Combination of both |
| Rare need to add potassium perchlorate (not FDA approved) |
Diagnostic Evaluation and Classification
Given the high incidence of thyroid dysfunction, patients treated with amiodarone should be screened for thyroid dysfunction by monitoring thyroid function tests (TFTs). The 2016 American Thyroid Association (ATA) Guidelines for Diagnosis and Management of Hyperthyroidism and Other Causes of Thyrotoxicosis suggest monitoring TFTs before commencing treatment and after the first 3 months following initiation of amiodarone, and at 3- and 6-month intervals thereafter. It is important to avoid making a diagnosis of thyroid dysfunction when testing early after amiodarone initiation, as it causes transient changes in TFTs in many patients. It causes these changes by inhibiting type 1 5′-deiodinase enzyme activity, thereby decreasing the peripheral conversion of T4 to T3 and reducing the clearance of both T4 and reverse T3 (rT3). Consequently, the serum levels of T4 and rT3 increase and the serum levels of T3 decrease by 20% to 25%. Subsequently, the feedback mechanism leads to thyroid-stimulating hormone (TSH) elevation that will increase thyroid hormone production in an otherwise healthy thyroid and the equilibrium is restored with normalization of TSH. These changes typically resolve within the first 3 to 6 months. Hence, the diagnosis of hypothyroidism should be made based on TFTs obtained after 3 to 6 months of amiodarone treatment. However, the diagnosis of AIT can be made earlier if the biochemical changes of thyrotoxicosis are present and they are overt, such as undetectable TSH and elevated T4/T3. Although patients may present with clinical features similar to any other form of thyrotoxicosis, there are clinical features that may be unique to AIT. The effect of amiodarone may prevent the occurrence of symptomatic tachycardia. Also, there is a higher chance of apathetic thyrotoxicosis where the typical sympathetic features of thyrotoxicosis are absent but the patient develops weight loss, muscle weakness, and worsening of cardiac function, a concern especially in elderly patients. Some patients may only present with worsening of fluid retention, congestive heart failure, recurrence of the underlying arrhythmia after a period of quiescence, or may develop a new arrhythmia, which should raise the concern for AIT.
LABORATORY EVALUATION
AIT presents with the typical laboratory features of thyrotoxicosis of suppressed TSH and elevated thyroid hormones T4 and/or T3. Subclinical thyrotoxicosis can also be present in mild cases where T3/T4 remain normal in association with suppressed TSH. As amiodarone has no effect on the serum concentration of thyroid hormone-binding globulin (TBG), changes in the levels of free T4 and free T3 mirror those for total T4 and total T3. It is important to rule out nonthyroidal illness or drugs (heparin, dopamine, glucocorticoids, and biotin) as the only cause of the thyroid laboratory abnormalities before making a diagnosis of AIT. Patients with AIT, especially those who are hospitalized, may also have concomitant nonthyroidal illness, which decreases T3 levels; hence those may be normal when there is a combination of AIT and nonthyroidal illness. The T3/T4 ratio, which tends to be higher in patients with autonomous hyperthyroidism as compared with destructive thyroiditis, is not very helpful in diagnosing AIT because of amiodarone-associated inhibition of T4 monodeiodination. Testing for autoimmune etiology should be performed because presence of TSH receptor antibodies suggests latent Graves’ disease, thus categorizing AIT as type 1; thyroid peroxidase (TPO) antibodies are often positive in type 1 AIT, but are not pathognomonic or necessary for this diagnosis ( Table 2.1 ). Interleukin-6 (IL-6) level has been deemed in the past to help with AIT diagnosis but the test is not routinely performed these days as many cases overlap in IL-6 values and the discrimination does not appear to be sensitive and specific enough.
RADIOLOGIC INVESTIGATIONS
Thyroid ultrasonography by itself has low diagnostic value in AIT, but when combined with color-flow Doppler sonography (CFDS), it provides a noninvasive real-time assessment of thyroid vascularity that can be very helpful in separating the AIT types. CFDS scores from 0 (absent vascularity) to 3 (very intense vascularity) are thus employed. Usually, type 2 AIT is characterized by absent thyroid gland vascularity (CFDS score 0) due to destructive thyroiditis, whereas type 1 AIT has normal to increased vascularity (CFDS score 1, 2, or 3) , ( Table 2.1 ). Even though CFDS is the best option in terms of diagnostic tests to classify AIT, it is not always helpful. , It has been reported that as many as 42% of patients classified as type 2 (absent vascularity) and 64% classified as type 1 based on CFDS may not respond to the respective therapy. The accuracy of thyroid ultrasonography with CFDS is also highly dependent on the operator skills and expertise. Thus whether the variability of interpreting CFDS has to do with the acquisition of the ultrasound images or to the presence of mixed AIT type remains to be determined. Twenty-four-hour iodine-123 or iodine-131 uptake of the thyroid has also been studied as a modality to classify the type of AIT. This will theoretically be very low or absent in type 2 AIT, whereas it can be low, normal, or high in type 1 AIT. However, in iodine-replete areas, especially with the iodine load from amiodarone, most cases of AIT have very low or absent uptake irrespective of the type; hence this diagnostic modality is not usually a useful distinguishing investigation. Other nuclear isotopes have been employed for their predictive nature in separating AIT types. The predictive nature of technetium-99m (99mTc)-sestamibi scintigraphy for AIT is low, and the numbers of patients reported as studied with these isotopes are small. , , Hence these methods have limited clinical validation in the diagnostic evaluation of AIT and should be reserved mainly for research studies.
SUMMARIZING CLASSIFICATION OF AMIODARONE-INDUCED THYROTOXICOSIS
An effort should be made to subtype AIT based on laboratory and radiologic investigations (usually CFDS); however, many cases demonstrated features of both ( Table 2.1 ) or may not respond to the respective therapies instituted based on the initial subtype. Hence no single investigation can accurately define the best treatment strategy, which is at least partially due to the presence of mixed forms of the disease. At the Mayo Clinic, we incorporated previously reported criteria , , as well as our clinical experience, in addition to information obtained from thyroidectomy surgical pathology, to classify AIT subtypes as below. We used the lower limit of normal for 24-hour radioactive iodine uptake in our population, as we have reported in previous publications.
- 1.
Type 1 AIT: nodular thyroid or diffuse goiter greater than 15 g or positive TSH receptor antibody titer; with normal/increased thyroid vascularity on CFDS or 24-hour radioactive iodine uptake greater than 8%.
- 2.
Type 2 AIT: normal thyroid or small diffuse goiter less than 15 g; with negative TSH receptor antibody titer and low thyroid vascularity on ultrasound CFDS.
- 3.
Mixed: nodular thyroid or diffuse goiter greater than 15 g or positive antithyroid antibodies; with low thyroid vascularity on ultrasound CFDS.
From the clinical standpoint, AIT can develop at any time during or even after the discontinuation of amiodarone because of the large deposits of this lipophilic medication in the adipose tissues, which explains its long half-life. Following prospectively 200 AIT patients, Tomisti et al. showed that type 1 AIT cases demonstrated significantly higher thyroid hormone levels and an earlier average onset of disease after amiodarone initiation as compared with type 2 (average of 3.5 months versus 30 months); hence higher severity and earlier onset of thyrotoxicosis may be used as additional criteria to subtype AIT as type 1.
Approach to Management
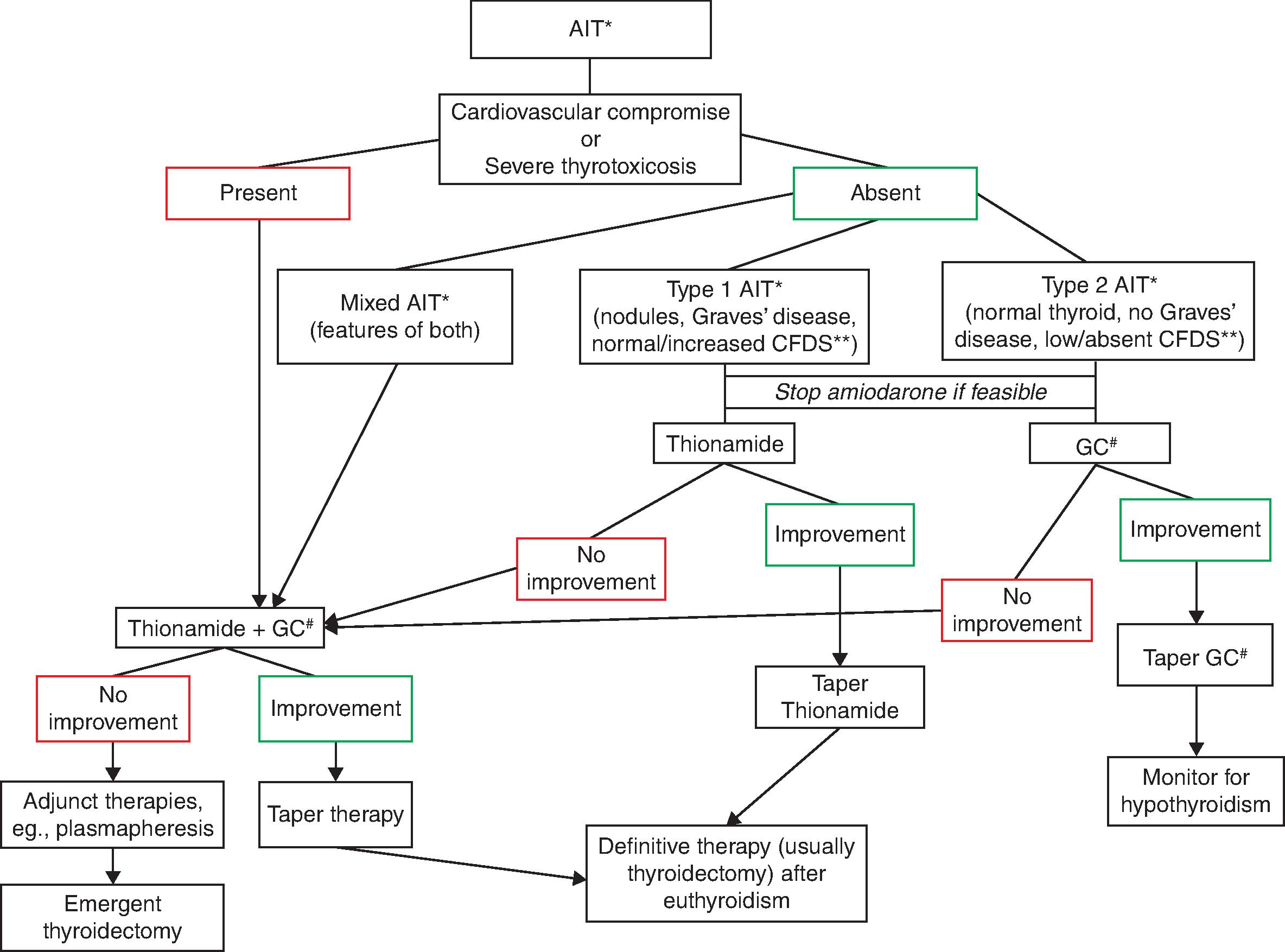
There is a degree of divergence between the experts regarding the importance of identifying the type of AIT ( Table 2.1 ) in order to guide its management. This is because the data on this issue are mixed, with some studies clearly supporting its role and others demonstrating that initial therapy choice and the subsequent response to it are not altered by the type of AIT. The 2016 ATA Guidelines for Diagnosis and Management of Hyperthyroidism and Other Causes of Thyrotoxicosis argue that as the pathogenesis of AIT is not fully understood, the classic division of AIT into two subtypes likely represents an oversimplification. They argue that many patients have mixed AIT forms, patients classified as type 1 or 2 often fail to respond to the subtype directed therapy, and, lastly, that some cases of type 2 AIT respond to measures not typically useful in destructive thyroiditis, such as perchlorate and oral cholecystographic agents. It is probably for this reason that several recent series did not attempt to classify the AIT subtypes. , , On the other hand, the European Thyroid Association (ETA) Guidelines for the Management of Amiodarone-Associated Thyroid Dysfunction propose that investigations should be performed to distinguish the subtype of AIT as best as possible so as to guide initial therapy. We consider it useful to utilize the clinical data and diagnostic tests for the purpose of AIT subclassification and subsequent therapy selection. Additionally, we emphasize the need for considering the preexistent cardiovascular comorbidities along with the severity of thyrotoxicosis in choosing the appropriate therapy for the patient. This therapy is then modified based on initial response ( Fig. 2.1 ). Thus, in our practice, we recommend that patients who are stable from a cardiovascular standpoint, and have definitive evidence supporting a distinct subtype of overt AIT, may be tried on appropriate monotherapy, either antithyroid drug (thionamide) or glucocorticoid ( Fig. 2.1 ). Because AIT, especially mixed and type 1, may present with severe thyrotoxicosis or thyroid storm, special consideration should be given to the intensity of antithyroid therapy and the necessary systemic support measures (discussed in Chapter 1, “Thyroid Storm: Acute Thyrotoxicosis”). However, the management of thyroid storm associated with AIT as compared with other causes of thyrotoxicosis is slightly different due to the lack of efficacy of iodine-based therapies.

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree