Frequently used abbreviations
AACE
American Association of Clinical Endocrinologists
AAES
American Association of Endocrine Surgeons
AHA
American Heart Association
APA
Aldosterone-producing adenoma
ARR
Aldosterone–renin ratio
AVS
Adrenal venous sampling
BAH
Bilateral adrenal hyperplasia (a.k.a. bilateral idiopathic hyperplasia, idiopathic hyperaldosteronism)
FH
Familial hyperaldosteronism
GRA
Glucocorticoid-remediable aldosteronism
HTN
Hypertension
JNC
Joint National Committee
PA
Primary hyperaldosteronism
Primary aldosteronism (PA) is the most common cause of secondary HTN. Detection and, therefore, reported prevalence have increased with education and screening. The prevalence of PA in the hypertensive population is estimated to be more than 10 % in recent studies, correlating with increased use of screening aldosterone–renin ratio (ARR) [5]. Furthermore, PA is correlated with the severity of hypertension, with upwards of 23 % prevalence in the resistant hypertension population and resultant worse cardiovascular outcomes [3, 6–8]. In comparison to primary hypertensive patients matched for blood pressure, patients with PA have four times increased odds of stroke, seven times increased odds of nonfatal myocardial infarction, and 12 times increased odds of atrial fibrillation [9].
PA is characterized by autonomous, inappropriately elevated plasma aldosterone. Unlike primary HTN, the hyperaldosteronism is nonsuppressible with sodium loading and can result in hypernatremia, hypokalemia, metabolic alkalosis, and suppression of renin [10]. There is a slight preponderance of males and no difference in incidence between race/ethnicities, and the mean age for diagnosis is 52 years for PA [11]. HTN alone is frequently the only sign of PA. In rare cases, patients can present with signs and symptoms of hypokalemia (i.e., muscle cramping, weakness) or patients may be diagnosed during biochemical evaluation of a nodule found on imaging as aldosterone-producing adenomas (APA) constituting 1 % of adrenal incidentalomas [12].
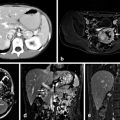
PA is most commonly caused by an APA (also known as Conn’s syndrome) or bilateral adrenal hyperplasia (BAH) [9, 10, 13–16]. In the largest prospective study to date, 1180 consecutive unselected hypertensive patients at 14 hospitals were assessed for PA. They found that 4.8 % had an APA and 6.4 % had BAH [8]. Furthermore, a higher proportion of APA among PA patients is reported at centers that perform adrenal venous sampling (AVS) , suggesting a significant rate of underdiagnosis of unilateral disease within the PA population with imaging alone [5, 8, 17]. A small subset of patients, less than 3 %, has unilateral hyperplasia which is diagnosed and treated using the same algorithm as APA with similar success rates [18]. Aldosterone-secreting adrenocortical carcinoma (ACC) occurs in 10.6 % of ACC [19].
Familial Hyperaldosteronism
Familial hyperaldosteronism (FH) is a group of autosomal dominant inherited diseases, consisting of three subtypes: (1) type I or glucocorticoid-remediable aldosteronism (GRA) is due to a CYP11B1/CYP11B2 chimeric gene at chromosome 8q22, (2) type II is due to an unknown mutation at chromosome 7p22, and (3) type III is due to mutations in the KCNJ5 gene at chromosome 11 which encodes a G-protein-gated potassium channel.
Type I FH or GRA has a prevalence of 0.66 % in patients with PA [20] and is the only form of PA in which aldosterone hypersecretion can be suppressed with glucocorticoids [21]. The CYP11B1 gene encodes for the 11-β hydroxylase isozyme that converts 11-deoxycorticosterone to corticosterone in the zona glomerulosa and CYP11B2 encodes for the 11-β hydroxylase isozyme that converts 11-deoxycortisol to cortisol [18, 22] . The mutation in patients with GRA fuses the promoter of CYP11B1 with the exon sequences of CYP11B2, resulting in a chimeric CYP11B1/CYP11B2 gene leading to: (1) an adrenocorticotropic hormone (ACTH)-dependent activation of the aldosterone synthase that converts corticosterone to aldosterone, and (2) an increase in the production of 18-oxocortisol and 18-hydroxycortisol [18, 22]. Normokalemia is characteristic in more than 50 % of GRA patients as aldosterone release is primarily under the influence of ACTH and not potassium [23]. Diagnosis of GRA should be made by genetic testing of selective PA patients who have the following criteria: (1) onset at a young age (e.g., < 20 years old), (2) family history of PA, and (3) cerebrovascular complications (e.g., hemorrhagic strokes due to ruptured intracranial aneurysms) at a young age (e.g., < 40 years old) [24]. Exogenous administration of glucocorticoids (e.g., dexamethasone) is considered the optimum therapy, while treatment with mineralocorticoid-receptor antagonists (MRA; e.g., eplerenone) is considered an effective alternative with less potential adverse effects [25].
Type II FH is the most common form of FH with 6 % prevalence in the PA populations [20]. Type II FN is an autosomal dominant inherited disease thought to be due to mutations located in chromosome 7p22 [26, 27]. Type II FH is clinically and biochemically indistinguishable from sporadic PA and as a result the diagnosis should be suspected in patients with a positive family history of PA [28]. There is no genetic test for type II FH available, and the same treatment protocols for PA patients should be followed. Blood-related family members should be screened for PA .
Type III FH is a rare condition, first described in 2008, associated with a number of mutations in the KCNJ5 gene encoding the G-protein-gated potassium channel Kir 3.4 [29–34]. These mutations lead to increased sodium conductance, which causes depolarization of glomerulosa cells and subsequent increased calcium [30]. Calcium entry signals aldosterone production and cellular proliferation, leading to hyperaldosteronism and massive hyperplasia of the adrenal glands. The clinical spectrum of the disease is broad, ranging from mild hypertension responsive to antihypertensive medications [31] to severe and resistant hypertension that requires bilateral adrenalectomy [34] . Certain mutations in the KCNJ5, as G151R, may be implicated with the more severe forms of type III FH [33]. Commercially available genetic testing is available for type III FH and patients presenting at a very young age or with family members with PA should be referred for screening. As with type II FH, the same treatment protocols for type III PA patients should be followed for the mild and moderate forms of type III FH. However, bilateral laparoscopic adrenalectomy may be required for severe forms of type III FH.
Diagnosis
Consensus guidelines vary on who should be referred for screening. While it is under debate, there are no current recommendations to screen all patients with hypertension for PA [11, 14, 15, 35]. Experts agree that patients with resistant hypertension (patients who remain above target blood pressure on three antihypertensive medications of which one is a diuretic) should be referred to a hypertension specialist to be assessed for secondary causes of hypertension [2–4]. Alternate forms of secondary hypertension and subtype differentiation should be considered (Table 2). Patients with hypertension with hypokalemia should be screened for both Cushing’s syndrome as well as PA. Lastly, patients who present with an incidentaloma found on abdominal imaging should undergo a functional workup. In rare cases, patients may present with a family history of PA or early-onset HTN or cerebrovascular accident.
Table 2
Physiological patterns of differential diagnoses
Disease | Potassium | Aldosterone | Renin |
|---|---|---|---|
Aldosterone-producing adenoma | 50 % ↓↓ | ↑↑ | ↓ |
Bilateral adrenal hyperplasia | 17 % ↓ | ↑↑ | ↓ |
Loop-diuretic therapy | ↓ | ↓ | ↑ |
Renal artery stenosis | ↓ | ↑ | ↑ |
Congenital adrenal hyperplasia | ↓ | ↓ | ↓ |
Cushing’s syndrome | ↓ | ↓ | ↓ |
Familial hyperaldosteronism | |||
Type I or GRA | Normal | ↑↑ | ↓ |
Type II | ↓ | ↑ | ↓ |
Type III | ↓ | ↑ | ↓ |
While serum potassium level (K+) has been cited historically as a screening blood test for PA, K+alone is inadequate as a screening test. In a recent study, hypokalemia only occurred in 9–38 % of a PA-confirmed population, more commonly in patients with an APA [5]. The Endocrine Society, European Society of Hypertension, American Association of Endocrine Surgeons (AAES) , American Association of Clinical Endocrinologists (AACE), and the American Heart Association (AHA) all advocate the use of the plasma ARR as the initial serological screening test for PA in hypertensive patients; however, threshold ratios and next steps in management differ (Table 3) [2–4, 36]. Some experts recommend use of a combination of tests. The AHA and AAES/AACE guidelines use an absolute serum aldosterone level ≥ 15 ng/dL in addition to an elevated ARR for diagnosis of PA. An ARR > 30 and a serum aldosterone > 20 ng/dL were shown to have both sensitivity and specificity > 90 % in diagnosing APA [37]. Rossi et al. developed a model using the combination of plasma renin activity (PRA), K+, and either serum aldosterone or captopril-suppressed aldosterone which they report has a superior diagnostic accuracy [38].
Table 3
Consensus guideline recommendations for PA screening
Guidelines | Criterion for screening | Suggested ARR threshold | Confirmatory test | Imaging | AVS |
|---|---|---|---|---|---|
AACE/AAES guidelines for the management of adrenal incidentalomas [12] | Incidental adrenal nodules + HTN, new HTN, HTN + hypokalemia, resistant HTN | ARR ≥ 20 + Plasma aldosterone ≥ 15 ng/dL | Oral salt loading or saline infusion testing | CT | Yes, in all except age < 40+ unilateral 1-cm nodule on CT (directly to surgery) |
American Heart Association guidelines for resistant hypertension [3] | Resistant hypertension | ARR 20–30 (using minimum renin 0.5 ng/mL/h) ± plasma aldo ≥ 15 ng/dL | – | – | – |
Endocrine Society clinical practice guidelines [4] | JNC 7 [2] stage 2, resistant HTN, hypokalemia, incidentalomas, family history | ARR 20–40 | Oral sodium, saline infusion, captopril, or fludrocortisone suppression testing | CT | Yes, in all surgical candidates |
ESH/SSC guidelines for the management of arterial hypertension | Incidental adrenal nodules + HTN, new HTN, HTN + hypokalemia, resistant HTN | ARR ≥ 20 + Plasma aldosterone ≥ 15 ng/dL | Oral salt loading or saline infusion testing | CT | Yes, in all except age < 40 + unilateral 1-cm nodule on CT |
More than setting a strict cutoff value (conventional threshold ARR ≥ 20), it is essential that the clinician be aware of the variability of results based on the setting, laboratory variations, and the various effects of antihypertensive medications on the renin–aldosterone axis. Age, posture, time of day, medications, K+, Na+, and renal function can all lead to false results [4]. See Table 4 for various factors affecting the ARR. Alternatively, patients can be screened with a 24-h urinary aldosterone on a high-sodium diet.
Table 4
Factors that affect the aldosterone–renin ratio (ARR)
Factor | Effect on ARR |
|---|---|
Increased age | FP |
Hypokalemia | FN |
Hypernatremia | FP |
Pregnancy | FN |
Renal failure | FP |
Resistant hypertension | FN |
Drugs | |
Diuretics | FN |
ACE inhibitors | FN |
ARBs | FN |
β-blockers | FP |
CCB | FN |
Confirmatory suppression testing is recommended with either oral (5-g sodium diet for three days, 24-h urinary aldosterone level > 12 mcg confirms PA) or intravenous saline load (2-L saline infused over 4 h, serum aldosterone > 10 ng/dL confirms PA), captopril, or fludrocortisone as the specificity of screening tests are low; however, caution should be taken as testing can be potentially dangerous for patients (i.e., exacerbation of congestive heart failure (CHF)) and withdrawal of antihypertensive medication is needed [35, 39]. For the most accurate test, patients on MRA (spironolactone or eplerenone) should be discontinued for 6 weeks prior to confirmatory testing, hypokalemia should be corrected, and hypertension controlled. However, screening with ARR has been shown to still be useful without withdrawing antihypertensives or when changing the regimen to those that affect the ARR less and should be utilized in cases where withdrawal of medications may be deleterious [4, 7, 11].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree