Aging of the Muscles and Joints: Introduction
Aging-related changes present in the tissues that make up the musculoskeletal system contribute to a number of common chronic conditions seen in older adults. In fact, musculoskeletal disease is the most common cause of chronic disability in people older than age 65 years. This is attributable both to the prevalence of diseases affecting the musculoskeletal system and the central role of the musculoskeletal system in physical function. Despite the prevalence of musculoskeletal disease, however, there is still much to learn about the pathogenesis of these diseases, including the role of aging in their development.
A number of diverse tissues, including muscle, tendon, ligament, cartilage, and bone comprise the musculoskeletal system; aging-related changes, as well as changes secondary to disuse, have been noted to occur in all of these. As in other systems, it is often difficult to separate aging from disuse from disease. Because the musculoskeletal tissues must function in concert for normal joint motion and thereby appropriate movement to occur, it is easy to see why physical function so commonly declines with age. However, it is also clear that regular submaximal stress to the musculoskeletal system through exercise can prevent or slow the age-related decline in physical function as well as improve function of diseased tissues.
There are a number of age-related changes common to tissues of the musculoskeletal system, which have also been noted in other tissues within the body (Table 112-1). Because of the relatively slow turnover rate of cells and matrix components in musculoskeletal tissues, aging-related changes such as the accumulation of advanced glycation end-products (AGEs) in matrix proteins and oxidative damage to cell and matrix components can have particularly profound effects on the aging musculoskeletal system. This chapter reviews aging in each of the major components of the musculoskeletal system including cartilage, muscle, ligaments and tendons, and intervertebral disks. Although bone is a key player in the musculoskeletal system, aging changes in this tissue are discussed in more detail in Chapter 117.
Accumulation of modified and degraded extracellular matrix components |
Increased collagen cross-linking by advanced glycation endproducts |
Decreased numbers of mesenchymal stem cells to replace lost cells |
Decreased mitogenic response to injury |
Decreased synthetic capacity in response to growth factor stimulation |
Articular Cartilage
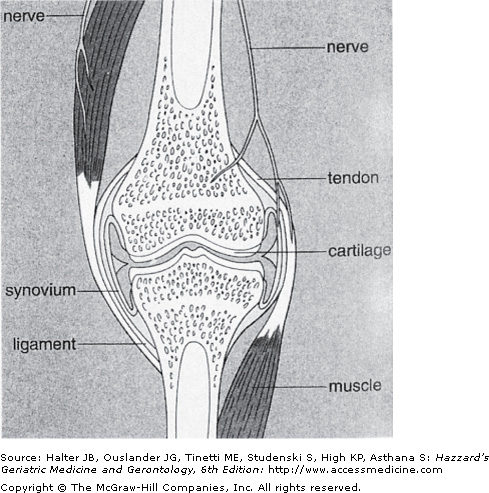
Articular cartilage is the tissue present on the ends of bones that comprise diarthrodial joints (Figure 112-1). This cartilage serves to provide a smooth surface with a very low coefficient of friction necessary for rapid, painless, and smooth joint motion. During joint motion, the opposing cartilage surfaces, separated by a thin layer of viscous and slippery synovial fluid, easily glide over each other. In addition to providing a form of lubrication for the joint surface, the synovial fluid also provides a large portion of the nutrition for the articular cartilage, which is an avascular and aneural tissue.
Figure 112-1.
Components of the musculoskeletal system that comprise the joint include bone, cartilage, muscle, tendon, ligament, synovium, and meniscus (not shown). Note that the nerve supply to the joint does not include cartilage, and, therefore, age- or disease-related changes in cartilage are not directly responsible for joint pain. (Reproduced with permission from Bullough PG. Atlas of Orthopedic Pathology, 4th ed. New York, New York: Gower Medical Publishing; 2004:9.4.)
When a load is placed across the joint surface, such as occurs in the knee joint during ambulation, the thin gel-like cartilage is compressed. Compression of the cartilage helps to distribute the load evenly to the underlying bone, where force can be absorbed. Cartilage is too thin to absorb any substantial amount of force itself. Rather, the force of joint loading is absorbed by the muscle and bone associated with the loaded joint. This concept is relevant to the pathogenesis of diseases occurring in weight-bearing joints. If changes occur in muscle or bone that limit their ability to absorb mechanical loads, then the articular cartilage may experience abnormal stresses, which could adversely affect the tissue. Loading of the articular cartilage in the knee is also modulated by the presence of the medial and lateral menisci, which absorb approximately 50% of the load placed on the tibiofemoral regions. Damage to a meniscus can therefore significantly increase the load placed on the cartilage.
Complete compression of cartilage during joint motion is resisted by forces within the tissue generated primarily by the highly negatively charged proteoglycans. Proteoglycans are composed of a core protein to which are attached chains of glycosaminoglycans containing negatively charged sulfate groups. In articular cartilage, a large proteoglycan called aggrecan is present. Aggrecan is bound at its amino-terminus to a long strand of hyaluronic acid. Many aggrecan molecules are bound to a single strand of hyaluronic acid to form a large complex that is quite hydrophilic. Cartilage is approximately 70% to 75% water, and much of this water is bound to the negatively charged groups on proteoglycans, forming a gel-like substance. Water is partially extruded from the cartilage matrix during compression, resulting in exposure of negative charges that, as they are brought closer together, resist further compression. As the force is released, the proteoglycans reexpand, and water is drawn back into the cartilage bringing along nutrients from the synovial fluid. The role of joint motion in providing cartilage nutrition may be very relevant to changes occurring in cartilage with disuse.
In addition to proteoglycans, a number of other matrix proteins are responsible for the physical properties of cartilage, including several types of collagen (predominantly types II, VI, IX, and XI) and glycoproteins (including fibronectin and cartilage oligomeric protein). Collagen is quite abundant in cartilage, comprising about half of the dry weight of the tissue. The majority of the collagen in cartilage is type II collagen. Type II collagen forms long fibrils that provide the tensile strength of the tissue, while the proteoglycans provide the resiliency. The collagen fibers appear to hold aggrecan complexes in place and prevent the proteoglycan gel from complete expansion, which results in the generation of swelling or osmotic pressure within the tissue. Thus, in normal cartilage, the osmotic pressure generated by proteoglycans is balanced by the tensile force provided by the collagen fibrils. The nonfibrillar collagens and the other matrix proteins in cartilage are thought to be involved in the organization and maintenance of the collagen fibrillar network, in structural interactions between collagen and proteoglycans, and in mediating interactions between the chondrocytes and the extracellular matrix (ECM).
Chondrocytes, the only cells present in cartilage, control the composition and organization of the cartilaginous matrix through the synthesis and degradation of selected ECM components. Together, the processes of synthesis and degradation result in ECM repair and remodeling. From studies performed in a number of tissues, including cartilage, it is becoming clear that ECM proteins can bind to cell surface receptors such as the integrin family of receptors, which, along with growth factors and cytokines, send signals to the cell to regulate matrix synthesis and degradation.
Several different types of enzymes capable of degrading cartilage ECM proteins are produced by chondrocytes, including the metalloproteinases, serine proteases such as elastase, and the cathepsins. The matrix metalloproteinases (MMPs) are prominent players in mediating cartilage catabolism. These include collagenases (MMP-1, MMP-8, and MMP-13), which cleave fibrillar collagens, the gelatinases (MMP-2 and MMP-9), which degrade denatured collagen, and stromelysin (MMP-3), which degrades proteoglycans. Other important MMPs in cartilage are the membrane-type (MT-MMPs), the ADAMs (a disintegrin and a metalloproteinase) and the ADAMTSs (a disintegrin and a metalloproteinase with a thrombospondin motif). The MT-MMPs are bound to the chondrocyte surface through transmembrane domains and can serve to activate other MMPs at the cell surface. An ADAM found in cartilage is ADAM-17, also known as tumor necrosis factor-α-converting enzyme (TACE), which functions to cleave tumor necrosis factor (TNF)-α to an active form. The ADAMTSs use the thrombospondin motif to interact with proteoglycans that can serve as substrates for these enzymes. ADAMTS-4 and ADAMTS-5 are aggrecanases that cleave the large proteoglycan aggrecan. The activity of the MMPs in cartilage is controlled in part by proteins called tissue inhibitors of metalloproteinase (TIMP). There is evidence for a reduction in the levels of TIMPs relative to the increased levels of MMPs in osteoarthritic (OA) cartilage.
A number of growth factors and cytokines are present in cartilage. These serve to regulate anabolic and catabolic processes involved in repair and remodeling (Table 112-2). In general, growth factors stimulate matrix synthesis, whereas the inflammatory cytokines inhibit synthesis and stimulate degradation. Insulin-like growth factor-1 (IGF-1), transforming growth factor-β (TGF-β), and osteogenic protein-1 (OP-1) appear to play a prominent role in regulating adult articular cartilage metabolism. They are expressed in cartilage and act in a paracrine and autocrine fashion to stimulate cartilage matrix synthesis as well as inhibit cytokine-stimulated matrix degradation. IGF-1 also appears to promote chondrocyte survival in conjunction with signals from the matrix. As discussed later in this chapter, there is growing evidence that chondrocytes from older individuals are less responsive to growth factor stimulation than those from younger persons. This could contribute to aging-related changes in the tissue, as well as to disease progression with age.
GROWTH FACTORS/CYTOKINES | ACTIVE |
|---|---|
IGF-I | Promotes cell survival, stimulates matrix synthesis |
OP-1 (BMP-7) | Stimulates matrix synthesis, inhibits some catabolic pathways |
TGF-β | Mitogenic, stimulates matrix synthesis, stimulates chondro-osteophyte production |
bFGF | Mitogenic, stimulates matrix synthesis, potentiates IL-1-induced protease release, may be more important in fetal and growth plate cartilage than adult |
IL-1β | Inhibits matrix synthesis and stimulates matrix degradation |
TNF-α | Similar to IL-1β |
LIF | Stimulates matrix degradation |
IL-6 | Inhibits proteoglycan synthesis |
IL-8 | Neutrophil chemoattractant |
MCP-1 | Monocyte chemoattractant; stimulates MMP production |
The mitogenic properties of growth factors noted in cell culture studies may not be relevant to normal adult cartilage homeostasis in vivo. In the normal tissue, adult articular chondrocytes do not appear to proliferate or do so at such low rates that proliferating cells are not detected. Chondrocytes, however, are not postmitotic cells. When removed from cartilage by enzymatic digestion of the tissue and placed in cell culture, human chondrocytes can proliferate. Proliferation of chondrocytes in situ occurs during the development of OA, when the presence of clusters or “clones” of chondrocytes is a common pathologic finding. These clusters of cells are thought to represent an attempt at repair of damaged and lost tissue (Figure 112-2).
Figure 112-2.
Normal (A) and osteoarthritic (B) cartilage. These sections from monkey knee joints are stained with toluidine blue, which binds to the negatively charged proteoglycans that are abundant in cartilage. In the osteoarthritic tissue, there is a loss of cartilage matrix staining, resulting from loss of proteoglycans. Other changes include fibrillation and cleft formation, presence of chondrocyte clusters, and a marked thickening of the subchondral bone. (Photographs are courtesy of Dr. Cathy S. Carlson.)
Normal articular cartilage in the adult contains fully differentiated cells and does not appear to contain its own source of undifferentiated mesenchymal cells available to repair or replace damaged or lost tissue. However, mesenchymal stem cells capable of differentiating into chondrocytes are present in the bone marrow, synovium, adipose tissue and periosteum. Current research is trying to use these cells for repair of cartilaginous lesions because endogenous repair responses in cartilage are inadequate to replace significant amounts of lost matrix, particularly after damage to the collagen network has occurred. The potential role of aging in the failed repair response in cartilage is discussed in the following sections.
Age-Related Changes in Cartilage
A number of studies have examined age-related changes in articular cartilage by using tissue collected from various animal species or human tissues obtained either at autopsy or from surgical specimens. When surgical specimens are studied, they usually are obtained from subjects undergoing hip or knee replacement surgery for arthritis, in which case the tissue is taken from grossly normal appearing areas but may not be truly normal. Tissues have also been obtained during hip replacement surgery after femoral neck fracture, which is usually associated with osteoporosis. In these cases, the cartilage is felt more likely to be normal because osteoporosis and OA tend to occur in different patient populations, although this is not always true.
Problems in interpreting the results of cartilage aging studies include translating the biology from lower species of animals to humans and separating disease effects from aging effects. In addition, a number of changes have been reported to occur in cartilage when comparing immature versus middle-aged animals. These changes are probably best considered to be developmentally related rather than aging-related. With the above caveats in mind, there do appear to be a number of changes occurring in cartilage that can be related to aging and, importantly, many of the aging-related changes can be contrasted to changes seen in disease (Table 112-3).
AGING | OSTEOARTHRITIS |
|---|---|
Decreased cartilage hydration | Increased cartilage hydration |
Proteoglycans | Proteoglycans |
Normal quantity | Decreased quantity |
Smaller size | Smaller size |
Ratio of CS 4/6* decreased | Ratio of CS 4/6* increased |
Collagen | Collagen |
Normal quantity | Decreased quantity |
Increased stiffness | Decreased stiffness |
Increased cross-linking | Cross-links lost during degradation |
Chondrocytes | Chondrocytes |
No or reduced proliferation | Increased proliferation |
Reduced metabolic activity | Increased metabolic activity |
No change in subchondral bone | Increased subchondral bone thinkness |
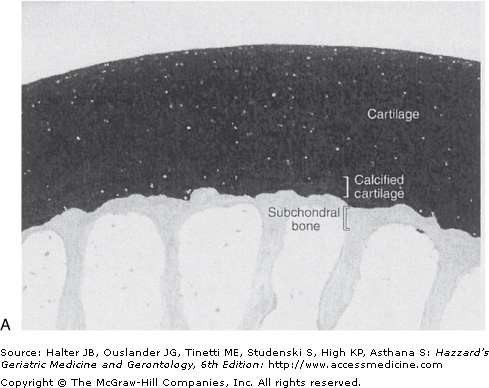
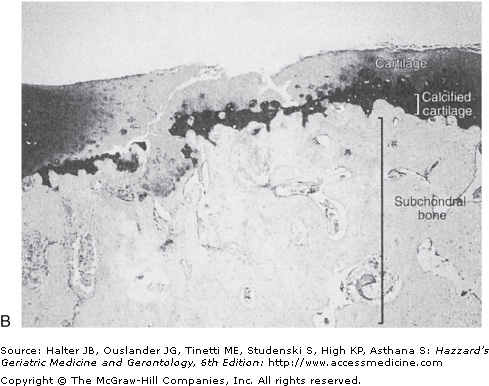
Structurally, fibrillation of the articular cartilage surface becomes more prevalent with age, particularly in the knee joint on the vertical ridge of the patella and in the tibiofemoral areas not covered by the menisci. These changes could represent the presence of early OA, although other pathologic changes of OA are not always present and it is most often asymptomatic. Morphologic changes become more common and more extensive with advancing age (Figure 112-3). Similar to the articular cartilage, age-related changes in the menisci have also been noted. Recent studies that have examined the knee joints of older adults using magnetic resonance imaging have noted that damage to the meniscus is strongly associated with damage to the articular cartilage in the same area and meniscal damage predicts further cartilage loss. This work highlights the importance of the meniscus to the normal function of the knee joint. Since the peripheral areas of the menisci contain nerve fibers, they may also be a source of pain in people with OA.
Figure 112-3.
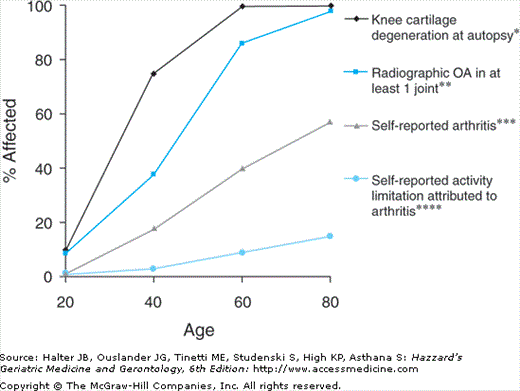
Effect of age on the prevalence of arthritis. *Knee cartilage degeneration at autopsy is the prevalence of significant histological changes of degeneration. **Radiographic evidence of OA (Kellgren and Lawrence Grade 2 or greater) present in at least one joint site (hands, feet, spine, knees, and hips) in a population survey in northern England. ***Self-reported arthritis and ****activity limitation attributable to arthritis derived from the National Health Interview Survey—US, 1989–1991. (Reproduced with permission from Loeser RF. Aging and the etiopathogenesis and treatment of osteoarthritis. Rheum Dis Clin North Am. 2000;26:547.)
There appears to be a slight decline in the number of chondrocytes present in cartilage with age. Early studies of cartilage from the femoral head noted a 30% fall in cell density between the ages of 30 and 100 years. But more recent studies of knee joints have noted much lower cell loss with normal aging in the range of 1% to 2%. Cartilage from older individuals has also been noted to have microcracks in the calcified layer. The significance of these cracks is not clear, but if they extend to the underlying subchondral bone, they could provide a mechanism for the exchange of cytokines and growth factors between the two tissues; and if vascular invasion occurs, they could mediate remodeling of subchondral bone, which is a characteristic feature of OA.
A consistent biochemical finding in cartilage is an age-related decrease in hydration. A decrease in hydration could explain evidence obtained from magnetic resonance imaging of knee joints showing that some thinning of knee cartilage occurs with age, particularly at the femoral surface, which is more evident in women than men. The decrease in cartilage hydration is likely related to changes with age in the proteoglycans that bind the majority of the water in cartilage. The total proteoglycan content does not appear to change significantly, instead changes in proteoglycan structure have been reported that could affect its biophysical properties. Aggrecan molecules become smaller with age and are structurally altered as the result of proteolytic modification in the core protein as well as changes in the length and abundance of the attached glycosaminoglycan chains. Hyaluronic acid, to which the aggrecan molecules bind to form large aggregates, is also decreased in size with age. In addition, proteolysis of the aggrecan core protein between the G1 and G2 domains results in increased levels of molecules bound to hyaluronic acid that contain only the G1 region and therefore lack the remainder of the aggrecan molecule necessary for normal function. The half-life for the free binding region is calculated to be as long as 25 years, consistent with its accumulation in cartilage with aging. By occupying and competing for space on the hyaluronic acid strands, the bound G1 domains may reduce the number of newly synthesized aggrecan molecules bound to hyaluronic acid.
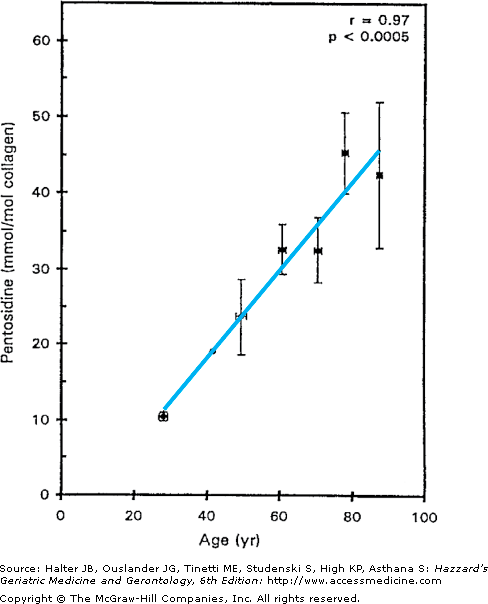
As with proteoglycans, there does not appear to be a significant reduction in the total amount of collagen present in cartilage with age. However, important changes in collagen structure and function have been noted. The collagen network appears to become stiffer with age. The increased collagen stiffness is thought to be a result of increased collagen cross-linking. There is evidence for nonenzymatic glycosylation in cartilage from older adults that result in the formation of pentosidine residues that can cross-link collagen molecules. An age-related accumulation of AGEs has been noted in cartilage (Figure 112-4). Pentosidine and other AGEs have been found in collagen, as well as in aggrecan. The accumulation of pentosidine is reported to be greater in collagen than aggrecan because of the exceptionally long half-life of collagen in cartilage, which is calculated to be approximately 117 years.
Figure 112-4.
Age-related accumulation of advanced glycation end-products in cartilage. Pentosidine levels were measured in cartilage samples from 36 donors clustered into 10-year age intervals. Results shown are mean ± SEM (standard error of mean). (Reproduced with permission from DeGroot et al. Age-related decrease in proteoglycan synthesis of human articular chondrocytes: The role of nonenzymatic glycation. Arthritis Rheum. 1999;42:1003.)
In addition to increased cross-linking from the accumulation of advanced glycation end products, collagen fibril diameter tends to increase with age, and this may also contribute to changes in collagen stiffness. Increased collagen network stiffness could contribute to the decrease in hydration, as stiffer collagen would tend to cause greater proteoglycan compression and thereby push out more water from the matrix. Biomechanical studies suggest that the stiffer network is more prone to fatigue failure. With age there is a decrease in the tensile strength of cartilage as well as a decrease in overall tensile stiffness. Therefore, age-related changes in the overall composition of the cartilage matrix result in a tissue that is less capable of handling mechanical stress.
In addition to changes in type II collagen and aggrecan, age-related changes in several of the other less-abundant cartilage matrix proteins have been reported. These include a decrease in type IX collagen, a protein that may be important in holding together adjacent collagen fibers and an increase in link protein, the protein that helps bind aggrecan molecules to hyaluronic acid. While link protein is increased with age, it also appears to undergo proteolytic modification with age that could affect its function.
Changes in the proliferative and synthetic capacity of chondrocytes with age have been noted. There is evidence for an age-related decreased mitogenic response to serum and growth factor stimulation. There is also evidence for telomere shortening in chondrocytes isolated from older adults but it is not clear if telomere dysfunction is the cause of the reduced mitogenic response. In addition, a reduction in proteoglycan and protein synthesis in response to growth factor stimulation has been in seen in cartilage from older animals, including nonhuman primates. Likewise, decreased proteoglycan synthesis in response to serum stimulation has been noted in human cartilage from older adults. In the human samples, the decreased serum response correlated with the presence of advanced glycation end-products. Other studies also suggest that a decline in cell signaling in response to growth factors is responsible for the decreased mitogenic and synthetic responses.
A reduction in response to growth factor stimulation with age, disease, or both, could be significant in the development of OA, where catabolic processes are greater than anabolic. In addition, there appears to be an age-related increase in the ability of catabolic factors, including the cytokine interleukin-1β (IL-1β) and a fibronectin matrix fragment, to stimulate MMP production by chondrocytes. These age-related changes could contribute to the anabolic–catabolic imbalance that has been observed in OA.
An age-related finding in cartilage, which is also observed in many other soft tissues, is an increased prevalence of crystals and calcification. It is difficult to determine the relative effects of age and disease on cartilage calcification. Calcification or crystal formation within cartilage is a common feature of OA, particularly in advanced disease and, like OA, age is the strongest risk factor for the development of crystal-associated arthritis. Cartilage from older individuals often contains crystals composed of calcium pyrophosphate dihydrate or hydroxyapatite. Studies with animal tissues show that the increase in the formation of calcium pyrophosphate crystals may be caused by an age-related increase in the activity of transglutaminase, an enzyme that is involved in the biomineralization process. Also chondrocytes from older individuals produce more inorganic pyrophosphate in response to TGF-β stimulation, despite a decreased proliferative response to this and other growth factors.