Aging of the Hematopoietic System: Introduction
Aging of the lymphohematopoietic system often manifests as a blunted response to hematopoietic stress and is thought to be associated with an increased incidence of neoplasia, autoimmune diseases, and infections in elderly people. Although the physiological basis of this suboptimal response remains unclear, our understanding of human hematopoiesis has increased exponentially over the last two decades. However, most of the initial breakthroughs, as well as the seminal findings in the biology of hematopoiesis, have occurred in murine models. This review will discuss (1) the biology of hematopoiesis, (2) age-related changes in lymphohematopoiesis, and (3) indications for the use of hematopoietic growth factors in elderly patients.
Biology of Hematopoiesis
The hematopoietic system derives from a small pool of hematopoietic stem cells (HSCs), which can either self-renew or differentiate along one of several lineages to form mature leukocytes, erythrocytes, or platelets. HSCs differentiate into mature cells through an intermediate set of committed progenitors and precursors, each with decreasing self-renewal potential and increasing lineage commitment. Hematopoiesis is tightly regulated by a complex series of interactions between HSCs, their stromal microenvironment, and diffusible regulatory molecules (hematopoietic growth factors) that effect cellular proliferation. The orderly development of the hematopoietic system in vivo and the maintenance of homeostasis require that a strict balance be maintained between self-renewal, differentiation, maturation, and cell loss.
HSCs cannot be directly observed, but are defined and identified by their ability to reconstitute and maintain hematopoiesis. The earliest morphologically recognizable cells of the myeloid and erythroid series are the myeloblasts and proerythroblasts (Figure 101-1). These cells are derived from morphologically unrecognizable progenitors that were first identified by in vitro culture techniques. There are two forms of erythroid progenitors: a more primitive precursor referred to as a burst-forming unit–erythroid (BFU-E), and a more mature progenitor referred to as the colony-forming unit–erythroid (CFU-E). A committed myeloid progenitor, also known as colony-forming unit–granulocyte/macrophage (CFU-GM), is the immediate precursor of the myeloblast. The committed progenitor cell compartments are supplied, in turn, by a common pluripotent stem cell, which has the capacity to differentiate into either hematopoietic or lymphoid cells. Figure 101-1 shows the hierarchy of cellular proliferation and differentiation in this pathway. The pluripotent HSC is called a colony-forming unit–spleen (CFU-S) by virtue of its ability (1) to produce colonies in spleens of lethally irradiated mice and (2) to repopulate the marrow of lethally irradiated recipients. There is evidence that the number of CFU-S in cell cycle is minimal, but that cycling can be greatly increased if demands for regeneration are increased. The CFU-S has a heterogeneous self-renewal capacity whereby an uncommitted CFU-S with high self-renewal capacity produces more committed CFU-S with decreasing self-renewal capacity and increasing differentiation potential.
Modern multichannel flow cytometry together with the availability of antibodies for different progenitor cell surface markers has validated the above findings, by allowing the identification of distinct progenitor cell populations with variable potential for hematopoietic reconstitution in lethally irradiated recipients. All HSC activity in many strains of mice is contained within a minor bone marrow population with a cell surface phenotype of c-kit positive (K), lineage negative (L), and Sca-1 positive (S), also known as the KLS cell fraction. However, only 1 in 30 KLS cells have true stem cell function. Two additional murine bone marrow markers, flk 2 and murine CD34, have also been identified. Thus, the KLS, flk2−, CD34− cells are the only cells within the KLS fraction, which possess long-term multilineage reconstituting HSC ability and are also known as the long-term repopulating stem cells or the LT-HSC. The KLS, flk2+, CD34+ cell fraction has limited repopulation potential and is known as the ST-HSC.
A major question with regard to the aging hematopoietic system is whether or not the pluripotent HSC has a finite replicative capacity. Studies of long-term bone marrow culture show that maintenance of hematopoiesis varies inversely with the age of the donor from which the culture was initiated. Additional studies using serial transplantation, whereby HSCs are subjected to in vivo serial transfer into recipient animals in which hematopoiesis has been ablated, also reveal a gradual loss in the ability to self-replicate. Thus, CFU-S from young donors is better able to repopulate the marrow of irradiated mice than CFU-S from old donors. A finite replicative capacity of the HSC was also found in an in vivo mouse model. Although finite, the lifespan of HSCs is thought to be well in excess of the potential lifespan of a species.
With the ability to detect stage-specific membrane markers, it is now possible to distinguish between long-term (LT) and short-term (ST) HSCs, and thus more accurately dissect the changes in the subtypes of CFU-S with age. Isolated bone marrow cells bearing the phenotype of LT-HSC and ST-HSC demonstrate a fivefold increase in the frequency of cells with the stem cell phenotype from older mice. However, their functional capacity was only a quarter of that seen with these cells from younger mice. The impact of aging on the lineage potential of stem cells has now been studied by transplanting limited numbers of purified LT-HSC from young and old mice into young congenic recipients, followed by analysis of donor cell contribution to B-cell, T-cell, and myeloid cell lineages at multiple time points posttransplant. These studies demonstrated that total donor reconstitution was consistently diminished in mice transplanted with old HSC. Furthermore, it was also noted that LT-HSCs from old mice had an increased propensity for myeloid differentiation and were deficient in generating mature lymphocytes. This skewed pattern of myeloid–lymphoid differentiation was not influenced by the aging marrow microenvironment, and was primarily thought to be an inherent attribute of the aging LT-HSC. Furthermore, in a murine model of chronic myeloid leukemia (CML), older animals with CML primarily developed a myeloid disorder with rare lymphoid involvement, as opposed to involvement of both myeloid and lymphoid lineages in younger animals with CML. Thus, it has been postulated that the myeloid dominance of adult leukemia may in part be driven by age-related deficient lymphopoiesis. Gene expression analysis of aging LT-HSCs supports this hypothesis by revealing (1) a 2- to 12-fold down-regulation of lymphoid lineage genes, (2) a two- to sixfold up-regulation of myeloid genes, and (3) a two- to fivefold up-regulation of leukemic proto-oncogenes like Aml 1, Pml, and Eto. In all, more than 700 genes were found to be differentially regulated with aging in the LT-HSCs, with genes involved in inflammatory and stress responses being up-regulated, and genes regulating chromatin remodelling and DNA repair being down-regulated.
The effect of age on committed HSC number and on the number of differentiated hematopoietic bone marrow cells has also been examined. In mice, no age-related reduction in the number of erythroid (BFU-E, CFU-E) or myeloid/macrophage (CFU-GM) progenitor cells occurs. Red blood cell survival is unchanged with aging, the plasma iron turnover and erythron iron turnover are unchanged, and the red blood cell mass is normal. The apparent anemia frequently observed in aged mice appears to be caused by an expansion of plasma volume. These findings and those of others indicate that no change in basal hematopoiesis occurs with aging. However, the ability of the aged hematopoietic system to respond to increased demands appears to be compromised. For example, older mice recover their hemoglobin values more slowly after phlebotomy than do young mice. Furthermore, when aged animals are placed in a high-altitude chamber, the expected increase in hemoglobin level is more variable and tends to be lower in older, as compared with younger, animals. Similar observations have also been made for the myeloid lineage, whereby bacterially challenged older mice undergo myeloid exhaustion earlier than younger mice.
The fragility of the aged hematopoietic system is further highlighted by studies on mice approaching their maximal life expectancy. When 48-month-old C57 BL/6 mice were housed in individual cages (one animal per cage), no change in hematopoiesis was seen. If, however, they were housed in groups of five animals per cage, the animals became more anemic and the number of stem cells in their bone marrow decreased. Thus, a suboptimal response in the face of stimulus-driven hematopoiesis and a failure to maintain homeostasis is a central characteristic of the aging hematopoietic system.
The mechanism/s responsible for the decline in HSC function remain unclear. Recently, accumulated DNA damage has been proposed as the principal and unifying mechanism underlying age-dependent HSC decline. HSC reserves and function with age in mice deficient in several genomic maintenance pathways including nucleotide excision repair (XPDTTD mice), telomere maintenance (mTR−/− mice) and nonhomologous end-joining (Ku80−/− mice) have been studied. None of the knock-out mice had a diminution either in HSC number or stem cell frequency. However, HSC functional capacity was severely affected under conditions of stress, leading to loss of reconstitution and proliferative potential, diminished self-renewal, increased apoptosis and, ultimately, functional exhaustion. Furthermore, endogenous DNA damage was noted to accumulate with age in wild-type HSCs. These data were thought to be consistent with DNA damage accrual being a physiological mechanism of stem cell aging that may contribute to the diminished capacity of aged tissues to return to homeostasis after exposure to acute stress or injury. Efforts are also underway to dissect the molecular mechanisms of HSC senescence. Analyses of cell cycle genes have implicated reciprocal roles for the cycle-dependent kinase inhibitors p21 and p18 in HSC function, with a deletion of p21 accelerating hematopoietic exhaustion and a deletion of p18 improving the engraftment potential of LT-HSC in serial transplantation experiments in mice.
The Effect of Age on Hematopoiesis in Humans
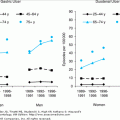
The human hematopoietic system derives from a small pool of stem cells that can either self-renew or differentiate along one of several lineages to form mature leukocytes, erythrocytes, or platelets. Marrow progenitors can be enriched on the basis of surface markers expressed at sequential stages of maturation. With respect to the myeloid lineage, the relevant surface markers are CD33 and CD34. CD33 is found on most cells of the myeloid lineage in the marrow, and CD34 is expressed only by more primitive progenitors (1% to 4% of the marrow cells). Thus, precursors of myeloid colony-forming cells (pre-CFC) express CD34 and lack expression of CD33 and other antigens expressed by mature lymphoid and myeloid cells. Since CD34+ marrow cells can engraft and reconstitute hematopoiesis in lethally irradiated baboons and humans, surface expression of CD34 on marrow and circulating cells serves as a surrogate for stem cell function.
Proliferation and differentiation of progenitor cells to become mature blood cells requires intimate contact between stem cells, stromal cells, and the extracellular matrix, and is thought to be mediated by the hematopoietic growth factors (HGFs). The HGFs are produced by multiple cell types and, on the basis of their actions, are characterized either as multilineage hematopoietins, e.g., stem cell factor (SCF), or mast cell growth factor (MGF), interleukin-3 (IL-3), and granulocyte-macrophage colony-stimulating factor (GM-CSF); or as lineage restricted hematopoietins, for example, granulocyte colony-stimulating factor (G-CSF), macrophage colony-stimulating factor (M-CSF), erythropoietin (EPO), thrombopoietin (TPO), and T-cell growth factor (IL-2). In addition to the above growth factors, lymphohematopoiesis is also modulated by an ever-expanding list of other cytokines (Table 101-1). These are produced by diverse cell types, have wide-ranging biological effects, and participate in a variety of cellular responses. Currently, G-CSF, GM-CSF, EPO, and IL-2 are in common clinical use.
LYMPHOKINE | MAJOR BIOLOGICAL PROPERTIES |
|---|---|
IL-1 | Activates resting T-cells; induces fever; activates endothelial cells and macrophages |
IL-2 | Growth factor for activated T-cells; synthesis of other lymphokines |
IL-3 | Supports growth of multilineage bone marrow stem cells |
IL-6 | B-cell growth factor |
IL-7 | B-cell and T-cell growth factor |
IL-12 | Differentiation of naive CD4 T cells to the Th1 subset |
Stem cell factor (SCF) | Promotes proliferation of primitive progenitors |
Granulocyte-macrophage colony-stimulating factor (GM-CSF) | Promotes growth of neutrophilic, eosinophilic and macrophage cell lineages |
Granulocyte CSF (G-CSF) | Promotes growth of neutrophilic cells |
Macrophage CSF (M-CSF) | Promotes growth of monocytes and macrophage |