Principles of Geriatric Endocrinology
As in other organ systems, the normal aging of the endocrine system is characterized by a progressive loss of reserve capacity, resulting in a decreased ability to adapt to changing environmental demands. This loss of homeostatic regulation reflects important alterations in hormonal synthesis, metabolism, and action, but these changes may not be clinically apparent under baseline conditions. In fact, basal plasma concentrations of many hormones and metabolic fuels are essentially unchanged with normal aging. This is illustrated by fasting plasma glucose levels that exhibit little change with normal aging, but after a glucose challenge, glucose levels increase much more in healthy older persons as compared to young adults. In some instances, the function of aging endocrine systems is maintained by compensatory changes in secretion of one hormone to offset the loss of function of another hormone in a feedback system, or to compensate for alterations in metabolic clearance. For example, in many older men with testosterone levels in the normal range, pituitary luteinizing hormone (LH) secretion and serum LH levels are increased (although levels usually remain within the normal range), partially offsetting a reduction in testicular testosterone secretion. However, in other cases, these compensatory mechanisms are inadequate to maintain normal function with aging even under basal conditions. For example, unlike cortisol, adrenal production of aldosterone and dehydroepiandrosterone (DHEA) declines disproportionately to clearance rates with aging, leading to age-related decreases in plasma levels of these hormones even under baseline conditions.
The presenting manifestations of endocrine disorders in older adults are often nonspecific, muted, or atypical. For example, hypothyroidism and hyperthyroidism may present similarly with nonspecific symptoms in older people, such as weight loss, fatigue, weakness, constipation, and depression. Endocrine diseases may also present with signs and symptoms that are classic for older patients yet atypical compared to those commonly observed in younger patients. As illustrations, thyrotoxic older patients may exhibit apathy and depression with psychomotor retardation (“apathetic hyperthyroidism”), and diabetes mellitus may present with hyperosmolar nonketotic state, a classic presentation rarely seen in individuals younger than age 50 years (see Chapters 108 and 109). In addition, with aging, it is increasingly common for illnesses to present without any appreciable symptoms, such as hypothyroidism or hypercalcemia secondary to hyperparathyroidism. Finally, the manifestations of endocrine disease may be altered or masked by coexisting illnesses and medications used to treat comorbidities that commonly occur in older people. For example, exacerbations of congestive heart failure or angina may be precipitated by hyperthyroidism in older patients with preexisting cardiac disease, but practitioners may mistakenly attribute the symptoms to worsening primary cardiac disease rather than to thyrotoxicosis in such patients.
Based on the above discussion, it is clear that a high index of suspicion for endocrine (and other) diseases is required in older patients with nonspecific signs and symptoms or functional decline. Indeed, the presence of these diseases may be appreciated only upon routine laboratory screening. However, there is no firm consensus on appropriate screening practices for endocrine disorders in asymptomatic older adults. Furthermore, even with normal aging, alterations in endocrine system function may have an important impact on the diagnostic evaluation of the older patient. For example, several factors contribute to an age-related decrease in intestinal calcium absorption, including decreased renal production of 1,25-dihydroxyvitamin D (1,25(OH)2D) and reduced intestinal responsiveness to 1,25(OH)2D. In turn, these alterations in vitamin D metabolism and action cause an increase in parathyroid hormone (PTH) levels to maintain calcium homeostasis, and ultimately a reduction in bone mass. As a result of these changes, together with the effects of sex hormone deficiency (a common problem in both older women and men), many asymptomatic older people require evaluation for osteoporosis (see Chapter 117). In addition, the presence of concomitant medical problems, medications, and changes in nutritional status and body composition may lead to a mistaken impression of endocrine disease in older as well as in younger people. For example, decreased serum triiodothyronine and thyroxine levels may occur in patients who are systemically ill but euthyroid (“euthyroid sick” syndromes), or thyroxine levels may be increased in others receiving certain medications such as estrogen supplements, falsely giving the impression of an endocrine abnormality (see Chapter 108).
Another factor complicating the evaluation of the older patient is the lack of age-adjusted normal ranges for most endocrine laboratory tests. Normal values for these tests are usually determined in healthy young subjects, or young to middle-aged blood donors, which may or may not reflect normal values in healthy older adults. Furthermore, most studies of aging and endocrine function are cross-sectional rather than longitudinal. Because interindividual variability and heterogeneity are important concomitants of aging, the usefulness of cross-sectional studies in predicting age-related changes within an individual is limited. Finally, normative studies in older populations are often confounded by the inclusion of subjects with age-associated diseases.
Alterations in the therapeutic approach for geriatric patients with endocrine disease are similar to those required in older people with illnesses involving other body systems. First, treatment plans must take into account coexisting medical illnesses, medications, alterations in clearance rate of hormones and medications, and changes in target organ sensitivity and effects. As a result of these factors, interventions such as hormone replacement may have different outcomes in older than in young persons. For example, data from the Women’s Health Initiative suggest that postmenopausal estrogen replacement therapy has a favorable benefit/risk ratio in many newly postmenopausal women, whereas in older women the risk of adverse effects such as coronary heart disease events tends to outweigh the benefits. Second, to minimize the risk of drug toxicity and polypharmacy, dosage levels for hormone replacement and medications must be adjusted for changes in clearance rate with aging, and patients should receive the lowest dose of medication needed to achieve the therapeutic effect. Third, new medications should be initiated at low doses and increased very gradually if needed. Fourth, the medication regimen should be reviewed periodically and medications should be discontinued if no longer needed. Finally, approaches that improve physical or cognitive function are of paramount importance for older people.
Neuroendocrine Regulation
Studies directly assessing parameters of hypothalamic neuroendocrine function have not been performed in vivo in humans. However, age-related effects on hypothalamic function may be studied indirectly by examining alterations in pulsatile secretion of pituitary hormones, which reflect changes in pulsatile secretion of hypothalamic releasing and/or inhibiting hormones. Other indices of the effects of aging on hypothalamic function in humans include pituitary hormonal responses to administration of (1) hypothalamic releasing hormones, (2) agents that block end-organ feedback (e.g., clomiphene and metyrapone), and (3) agents that stimulate hypothalamic/pituitary hormonal secretion (e.g., the use of hypertonic saline to stimulate antidiuretic hormone [ADH] secretion, or arginine to stimulate growth hormone [GH] secretion).
A number of neurotransmitters and neuropeptides within the central nervous system (CNS) have been found to affect the secretion of hypothalamic and pituitary hormones. Changes in activity of these neurotransmitters and neuropeptides are responsible for many age-related alterations in the function of the endocrine system.
Dopamine released by hypothalamic neurons inhibits pituitary prolactin secretion. In turn, prolactin stimulates hypothalamic dopaminergic neurons, forming a short feedback loop. In humans, to a greater extent than some of the other hypothalamic neurotransmitters, changes in dopamine secretion are indirectly observable with commonly available methods. This is because dopamine is the major regulator of prolactin secretion, and changes in hypothalamic dopamine activity can be inferred through observation of changes in pulsatile prolactin secretion. The frequency of pulsatile prolactin secretion is unchanged with aging, suggesting an intact pulse generator. However, humans become relatively hypoprolactinemic with normal aging. Furthermore, the circadian rhythm of prolactin secretion is altered in older men, with a blunted or absent nocturnal rise in prolactin and reduced amplitude of prolactin pulses, as compared to young controls. Metoclopramide, a dopamine antagonist, increases nocturnal prolactin secretion to a greater degree in older people than in young subjects, suggesting that this blunted nocturnal elevation in prolactin in older men may be caused by an increase in dopaminergic tone with aging. In turn, these age-related alterations in dopaminergic activity may contribute to alterations in secretion of other anterior pituitary hormones. For example, after administration of the dopamine precursor l-dopa, plasma gonadotropins increase and thyroid-stimulating hormone (TSH) levels decrease in old but not in young male subjects, implying altered dopaminergic regulation of these hormones with aging.
Studies of postmortem brain tissue in normal older persons demonstrate a modest decrease in the number of noradrenergic neurons in the locus coeruleus, the primary source of noradrenergic innervation within the CNS. However, levels of norepinephrine (NE) in the cerebrospinal fluid (CSF) of normal older subjects are increased as compared to young adults, both in the basal state and in response to yohimbine, a stimulator of central noradrenergic activity. CSF NE is thought to be produced by noradrenergic neurons within the CNS rather than the peripheral sympathetic nervous system. Taken together with the observation that NE clearance from the CNS is unchanged in older adults, these findings suggest that central noradrenergic neuronal activity is increased with aging.
The noradrenergic neuronal system is thought to exert an important influence on pituitary secretion of a number of hormones, including GH, TSH and LH; therefore, an age-associated increase in central noradrenergic tone may be an important factor underlying age-related changes in the secretion of these hormones.
Endogenous opioid peptides are believed to be important regulators of neuroendocrine systems. The products of proopiomelanocortin metabolism are the most extensively studied of these peptides, including adrenocorticotropic hormone (ACTH), β-endorphin, β-lipotropin, and α-melanocyte-stimulating hormone. β-Endorphin, for example, is thought to exert a tonic inhibitory influence on gonadotropin-releasing hormone (GnRH) secretion and to be an important regulator of reproductive function. In older men, administration of the opioid receptor antagonist naltrexone causes a smaller increase in LH levels than it causes in young men, and unlike in young men, there is no increase in LH pulse frequency. Taken together, these observations suggest a decrease in central opioid tone with aging. However, in the periphery, basal plasma levels of β-endorphin are unaffected, and basal ACTH levels are unchanged or increased with normal aging. Furthermore, plasma ACTH, cortisol and β-endorphin responses to physostigmine, a drug that increases CNS cholinergic activity, and ACTH and cortisol responses to administration of corticotropin-releasing hormone (CRH), are increased in older compared to younger adults. Increased responsiveness of β-endorphin and the hypothalamic–pituitary–adrenal (HPA) axis with aging may alter the function of other endocrine systems.
Melatonin is a hormone produced in the pineal gland that is involved in the organization of circadian and seasonal biorhythms. Its synthesis and release are stimulated by darkness and inhibited by light. Therefore, the diurnal rhythm of melatonin secretion mirrors the day–night cycle. This circadian rhythm of melatonin production is controlled by a “pacemaker” in the suprachiasmatic nucleus of the brain, but changes in environmental lighting can alter the timing of this rhythm. For example, light exposure inhibits melatonin release in a dose-dependent fashion and it is possible to reverse the diurnal melatonin rhythm within a few days by reversing the diurnal pattern of light exposure. Melatonin affects reproductive function in a variety of species, with antigonadotropic effects that are more pronounced in species with marked seasonal variation in reproductive function and breeding patterns. However, even in humans dwelling in the Arctic, conception rates and pituitary–gonadal function are reduced during the winter as compared to the summer. Melatonin levels are increased in women with hypothalamic amenorrhea, and there has been some interest in very-high-dose melatonin (75–300 mg) as a potential contraceptive agent, used in combination with a progestin.
During development and aging, melatonin production is negligible in young infants, but rises markedly between 3 months and 1 to 3 years of age, when nighttime levels are at their highest. After early childhood, melatonin secretion appears to gradually decrease, with a progressive decline in melatonin secretion by the age of 30 years. Nocturnal and 24-hour melatonin secretion appears to be similar in healthy older men and women free of confounding conditions and medications, as compared to healthy young men. Additionally, MT1 melatonin receptor expression is reduced in suprachiasmatic nucleus neurons of aged subjects and to a greater extent in persons with Alzheimer’s disease, compared to young adult subjects.
Melatonin has long been known to have sedative effects, suggesting a role in sleep production. A meta-analysis of 17 studies of the effects of exogenous melatonin on sleep in subjects of various ages showed improvement in several parameters of sleep quality. In older people with insomnia, serum melatonin levels were lower than in age-matched controls without insomnia, suggesting that insomnia in older adults is caused, at least in part, by melatonin deficiency. Older adults are often exposed to significantly less environmental light than younger adults, a factor that is associated with diminished nocturnal melatonin secretion. Supplementary exposure to bright light during midday increased melatonin secretion in older adults, and tended to improve sleep disturbances in older insomniacs. In older insomniacs, short-term administration of melatonin in doses of 0.1 to 6 mg per day improved some measures of sleep quality. In older people without sleep complaints, melatonin improved sleep quality in some studies but not others. No long-term studies of melatonin supplementation have been performed in older subjects. Ramelteon, an MT1/MT2 melatonin receptor agonist, improved some measures of sleep quality in older people with chronic primary insomnia, and is approved by the U.S. Food and Drug Administration for improving sleep in individuals with difficulty falling asleep and has not been associated with dependence.
Melatonin is a potent free radical scavenger and it has been proposed that if the age-related decline in melatonin were prevented, then potentially the degenerative changes of aging could also be delayed. However, others have noted that the reported antioxidant effects of melatonin probably occur only at pharmacologic concentrations. In humans, high-affinity melatonin receptors are present on CD4 T lymphocytes and mononuclear cells in peripheral blood synthesize melatonin. Moreover, inhibition of serotonin-N-acetyltransferase, the enzyme catalyzing melatonin synthesis, was reported to cause a significant decrease in interleukin-2 (IL-2) secretion that was restored by melatonin administration. It has been proposed that melatonin may be an intracrine and paracrine regulator of immune function.
Based in part on the assumption that a decline in endogenous hormone levels constitutes a deficiency needing replacement, there has been considerable enthusiasm in the lay community for supplementation of certain hormones, including melatonin. Melatonin is readily available without a prescription in the United States, and many older people take melatonin without physician consultation. It is noteworthy that melatonin dosages of 1 to 5 mg contained in preparations commonly available over-the-counter boost nighttime levels 10 to 100 times higher than the usual maximum within an hour after administration, and only very low doses (0.1–0.3 mg) achieve peak serum levels within the normal nighttime range. Thus far, no serious side effects have been reported in humans after melatonin ingestion (aside from sleepiness after the dose), even with pharmacologic melatonin doses. However, large, long-term studies of melatonin use are still lacking, and the long-term risks and benefits of melatonin supplementation remain to be determined. Until this information is available, it is premature to advocate its use.
Alzheimer’s disease is characterized by a profound impairment of cholinergic neuronal function within the CNS, but changes in the function of many other neurotransmitter and neuropeptide systems have also been described. In turn, these CNS alterations are associated with changes in pituitary and peripheral hormone levels. One important endocrine system abnormality observed in patients with Alzheimer’s disease is an increase in HPA axis activity with elevated plasma ACTH and cortisol levels, and lack of suppression of cortisol levels by dexamethasone, suggesting decreased glucocorticoid feedback inhibition. In addition, ACTH responses to exogenous administration of CRH are blunted, which may be a result of down-regulation of pituitary responsiveness to CRH secondary to chronically increased hypothalamic CRH stimulation. Elevated cortisol levels are associated with memory impairment in older nondemented people, and appear to predict subsequent decline in memory function. Finally, cortisol and ACTH responsiveness to physostigmine, which increases central cholinergic activity, is markedly increased in patients with Alzheimer’s disease and normal older persons, as compared to young subjects.
This elevated glucocorticoid milieu may play a role in the pathogenesis of Alzheimer’s disease. It has been hypothesized that chronically increased glucocorticoid stimulation damages the hippocampal neurons that mediate glucocorticoid feedback inhibition of the HPA axis, leading, in turn, to a “glucocorticoid cascade” of neurodegeneration in Alzheimer’s disease (and to a lesser extent in the aging brain). While controversial, this hypothesis remains under active study to determine whether glucocorticoid levels are a potentially modifiable risk factor for Alzheimer’s disease.
Numerous epidemiological studies indicate a link between type 2 diabetes mellitus and increased risk of Alzheimer’s disease. In normal physiology, and when exogenous insulin is administered at optimal doses, insulin appears to enhance some aspects of memory. However, the presence of insulin resistance as in the metabolic syndrome and type 2 diabetes mellitus is associated with an increased risk of cognitive dysfunction and possibly of Alzheimer’s disease. Insulin might play a role in the pathophysiology of Alzheimer’s disease through a number of mechanisms. For example, insulin may increase levels of beta-amyloid, the primary component of senile plaques, by competing with beta-amyloid for insulin-degrading enzyme, which catalyzes the breakdown of both peptides within the CNS. Insulin-degrading enzyme has a high affinity for insulin, so under conditions of high insulin availability in the brain insulin degradation is favored over degradation of beta-amyloid, thereby potentially promoting plaque formation. Additionally, increased insulin may promote cerebral inflammation, which is thought to be part of the pathophysiology of Alzheimer’s disease. Inflammatory markers such as C-reactive peptide and interleukin-6 are elevated in older people with insulin resistance, and peripheral administration of high doses of insulin under hyperinsulinemic-euglycemic clamp conditions increases levels of proinflammatory cytokines within the CNS.
Many other alterations in hormone, hormone receptor, neuropeptide, and neurotransmitter levels have been reported in plasma, CSF, and postmortem brain tissue specimens in Alzheimer’s disease. However, such changes have not generally controlled for the effects of nutritional status, medications, or coexisting medical illnesses and depression. NE levels in CSF are increased both in individuals with Alzheimer’s disease and in normal older people. This increase is apparently caused by an increase in NE biosynthesis without a change in NE clearance. Because there is a marked loss of noradrenergic neurons in the locus coeruleus of the brain in patients with Alzheimer’s disease, these findings suggest compensatory activation of the remaining noradrenergic neurons in these patients.
Healthy aging is associated with a decrease in prolactin secretion in both men and postmenopausal women, reflecting a decrease in both basal prolactin secretion and the amplitude of pulsatile prolactin release. However, serum prolactin levels may vary considerably as they can increase in many situations including stress, exercise, and sleep. Mild hyperprolactinemia observed in some older adults may result from any of several underlying causes (Table 107-1), including hypothalamic diseases that interfere with the production of dopamine (prolactin-inhibitory factor). A number of medications commonly used by older adults inhibit dopaminergic activity and increase prolactin levels, and primary hypothyroidism increases thyrotropin-releasing hormone (TRH), which stimulates prolactin secretion.
Hypothalamic diseases |
Tumors |
Metastatic disease |
Meningioma |
Glioma |
Granulomatous diseases |
Sarcoidosis |
Tuberculosis |
Irradiation |
Primary hypothyroidism |
Pituitary stalk section |
Suprasellar extension of pituitary adenoma |
Trauma |
Pituitary prolactinoma |
Chronic renal failure |
Chronic liver disease |
Chest wall lesions |
Herpes zoster |
Surgery |
Drugs |
Phenothiazines |
Cimetidine |
Opiates |
Metoclopramide |
Estrogens |
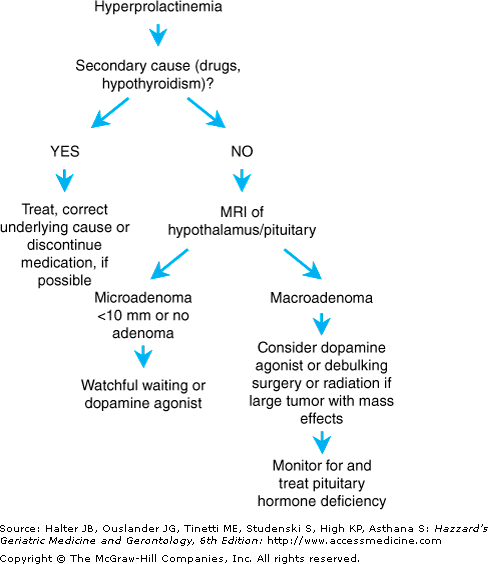
The clinical manifestations of hyperprolactinemia are typically subtle and often unrecognized. Hyperprolactinemia causes secondary hypogonadism and may exacerbate sexual dysfunction and cause gynecomastia. Rarely, galactorrhea may occur. In addition, hyperprolactinemia can accelerate age-related bone loss primarily because of the antigonadotropic effects of prolactin and resulting hypogonadism. Thus, measurement of prolactin is important in the evaluation of secondary causes of osteoporosis, especially when hypogonadism is present. The diagnostic approach for older adults with hyperprolactinemia is summarized in Figure 107-1. If drugs and hypothyroidism have been ruled out as causes of hyperprolactinemia, it is appropriate to obtain magnetic resonance imaging (MRI) to rule out the presence of a pituitary tumor or hypothalamic lesion (computerized tomography is much less sensitive.). Dynamic pituitary testing, such as a TRH test, is of no value in the diagnosis of this disorder.
If the hyperprolactinemia is secondary to another disorder such as hypothyroidism or a medication, the underlying cause should be corrected or the offending medication should be discontinued if possible, which often results in normalization of prolactin levels. However, if the etiology is a microadenoma (defined as a pituitary tumor <10 mm in size) or idiopathic hyperprolactinemia, options include watchful waiting or dopamine agonist medications. The natural history of microadenomas is incompletely understood, especially in older adults, but most prolactin-producing microadenomas are stable or involute with time. Accordingly, observation is the most appropriate strategy in many patients although treatment may be indicated if sexual dysfunction or additional risk factors for osteoporosis are present. Dopamine agonists such as bromocriptine or cabergoline are effective in reducing prolactin levels and normalizing reproductive axis function in most patients, but gastrointestinal, behavioral, and other side effects such as hallucinations are common in older patients. These side effects can be minimized by following a “start low, go slow” approach when beginning these medications. Of note, high doses of cabergoline may be associated with an increased risk of valvular heart disease. Surgery is rarely indicated, but may be necessary in those with macroadenomas (>10 mm) and persistent visual field defects or inability to tolerate dopamine agonists. Alternatively, radiation therapy may be an option for patients with large tumors who are unable to tolerate medical therapy and are not surgical candidates. However, the clinical response to radiation is often delayed for months to years, and anterior pituitary insufficiency eventually occurs in many, if not most, of these patients.
Age-associated alterations in secretion of other anterior pituitary hormones are discussed below, under the individual hormonal systems, and in Chapter 108 (Thyroid Disorders) and Chapter 46 (Menopause and Midlife Health Changes).
Although the incidence of pituitary tumors increases with aging, symptomatic pituitary tumors are uncommon even in people of advanced age. However, the prevalence of pituitary tumors at autopsy ranges from 8.5% to 27%, indicating that the vast majority of these tumors are clinically “silent.” In case series of pituitary tumors in older adults, the majority of lesions are apparently nonfunctioning adenomas, whereas 9% to 17% are GH-secreting tumors and 4.5% to 10% are prolactinomas. However, many, if not most, apparently nonfunctioning adenomas actually produce quantities of gonadotropins or the α-subunit of these glycoprotein hormones.
Nonsecreting tumors and tumors that secrete LH, follicle-stimulating hormone (FSH), or α-subunit are usually large at the time of diagnosis, because there are few or no symptoms of hormone overproduction. Gonadotropin hypersecretion from such tumors usually does not result in excessive gonadal hormone secretion although LH hypersecretion occasionally results in elevated testosterone levels. Clinical manifestations of these tumors are usually a result of a mass effect, including visual field abnormalities caused by compression of the optic chiasm, headaches, and panhypopituitarism. Gonadotropin-secreting pituitary tumors are particularly difficult to diagnose in postmenopausal women in whom gonadotropin levels are elevated as a result of ovarian failure. For these women, exaggerated α, LH-β, and FSH-β subunit responses, and/or intact gonadotropin responses to TRH are useful in diagnosing these tumors. However, pituitary “incidentalomas” are being increasingly identified during neuroimaging evaluations for comorbid conditions, accounting for 5% to 15% of pituitary tumors identified in older adults. Most of these are asymptomatic though endocrine and visual disturbances should be sought in these patients. Diagnosis of pituitary tumors depends on neuroimaging (especially magnetic resonance imaging). Management of large pituitary tumors usually involves transsphenoidal decompression and debulking, along with assessment of anterior pituitary hormone function and replacement of hormone deficiencies. Macroprolactinomas may be managed with dopamine agonists in many cases even when large or associated with visual field changes although these agents may be less effective in older than in young adults (Figure 107-1). Pituitary function should be reassessed in patients started on dopamine agonists for a prolactinoma because recovery of pituitary function occurs in some individuals treated with these medications.
As a pituitary macroadenoma grows, destruction of normal surrounding pituitary tissue occurs, leading ultimately to a predictable loss of gonadotropins and GH followed by TSH and eventually ACTH secretion. The resulting panhypopituitarism may present clinically as a constellation of symptoms including supine and/or postural hypotension, fatigue, loss of libido, weight loss, hypogonadism, hyponatremia, and hypoglycemia. Many of these symptoms are nonspecific and common in older patients without panhypopituitarism, so a high index of suspicion is required to make the diagnosis. Causes of hypopituitarism in adults are summarized in Table 107-2.
Pituitary adenomas |
Sequelae of irradiation or surgery for pituitary tumors |
Peripituitary tumors (e.g., meningioma, glioma, metastatic cancer) |
Vascular conditions (e.g., pituitary infarction, carotid artery aneurysm, subarachnoid hemorrhage) |
Traumatic brain injury (e.g., stalk section) |
Infiltrative or granulomatous diseases (e.g., lymphocytic hypophysitis, hemochromatosis, histiocytosis X, sarcoidosis) |
Infections (e.g., tuberculosis, mycoses, abscesses) |
Initially, measurements of basal serum levels of morning cortisol and ACTH; total or free testosterone (in men) and gonadotropins; free thyroxine and TSH; IGF-1; and prolactin may be sufficient to confirm panhypopituitarism. Measurement of both the pituitary hormone and the target hormone concentration allows assessment of the appropriateness of both levels together. Dynamic ACTH (cortrosyn) stimulation testing is indicated to confirm the diagnosis of secondary hypoadrenalism. ACTH and other dynamic tests of adrenal function are discussed below under “Laboratory Testing of HPA Axis Function” (also see Chapter 108).
As computed tomography (CT) and MRI have come into widespread use, it is increasingly common to discover incidentally the presence of both pituitary masses and empty sella syndrome. MRI studies of healthy young and older subjects found that pituitary height and volume tend to decrease with aging, with empty sella occurring in 19% of subjects. No relationship was found between pituitary volume and anterior pituitary hormone levels. Most cases of primary empty sella syndrome (i.e., not associated with prior pituitary tumor, surgery or irradiation) occur in obese, hypertensive middle-aged women. Clinically apparent pituitary dysfunction is uncommon, although up to 15% of subjects may manifest endocrine dysfunction including hyperprolactinemia, hypogonadism, or panhypopituitarism. However, the functional significance of an empty sella as an incidental finding on imaging studies in apparently healthy older patients is unclear. In such patients, conservative management is appropriate, including visual field testing and serum hormone testing to rule out subclinical pituitary dysfunction and suprasellar involvement.
Antidiuretic hormone (ADH), or arginine vasopressin (AVP), is synthesized in “magnocellular” neurons of the hypothalamus and released into the systemic circulation from nerve terminals in the posterior pituitary gland in response to increased plasma osmolality, decreased arterial pressure, and reductions in circulating blood volume. In addition, ADH synthesized in “parvocellular” hypothalamic neurons is released into the hypothalamic–pituitary portal circulation, in turn stimulating release of ACTH from the anterior pituitary into the systemic circulation. ADH appears to be colocalized with corticotrophin-releasing hormone (CRH) in parvocellular neurons of the hypothalamus. Administration of ADH together with CRH amplifies the effect of CRH on ACTH release, suggesting a role for ADH in responses to stress.
Older people are at higher risk than young adults for the development of both volume depletion and excess free water states. This is a result of age-related changes occurring in the systems that maintain volume status and osmolality, including ADH secretion, osmoreceptor and baroreceptor systems, renal concentration of urine and hormone responsiveness, and thirst mechanisms. There is evidence for a state of relative ADH excess with aging, with normal to increased basal ADH levels, increased ADH release after an osmotic stimulus such as hypertonic saline infusion, and impaired ethanol inhibition of ADH secretion in older persons, as compared with young adult subjects. Furthermore, renal free water clearance decreases with aging in proportion to declining glomerular filtration rate (GFR). With a reduction in GFR, proximal renal tubular fluid absorption is increased, leading to a decrease in fluid delivery to the distal diluting portions of the nephron. The net result is a decrease in the diluting capacity of the kidney and a decreased ability to excrete a free water load. This age-related reduction in free water clearance, together with relative ADH excess and the increased prevalence of conditions such as congestive heart failure, hypothyroidism, and the use of sulfonylurea and diuretic medication all contribute to the common occurrence of free water excess and hyponatremia in older people. When present, hyponatremia in frail older adults is usually mild and asymptomatic, but these individuals may develop more marked symptomatic hyponatremia in the setting of acute illness.
In contrast to the increased osmoreceptor sensitivity to ADH, baroreceptor regulation of ADH is decreased with aging, resulting in decreased ADH release in response to hypotension or hypovolemia and increased risk of volume depletion. Furthermore, renal responsiveness to ADH is diminished with aging, possibly because of chronic exposure to elevated ADH levels, leading to a decrease in maximal urine concentrating ability. Decreased aldosterone and increased atrial natriuretic hormone activity with aging contribute to the potential for volume depletion by decreasing the capacity of the kidneys to conserve sodium under conditions of fluid deprivation (see “Renin–Angiotensin–Aldosterone System” later in this chapter). Finally, healthy older adults exhibit decreased thirst and reduced fluid intake in response to fluid deprivation, hyperosmotic stimuli, and hypovolemia, further contributing to the risk of dehydration with aging. Older patients with immobility or dementia, such as nursing home residents, are at particularly high risk for severe dehydration and hypernatremia.
A number of factors predispose older people to nocturnal polyuria. These include age-related blunting of the circadian rhythm of circulating ADH, with loss of the nocturnal increase in ADH levels, and an increase in nighttime plasma atrial natriuretic hormone levels. Patients with Alzheimer’s disease have lower circulating ADH levels than cognitively intact people of the same age, and appear to be particularly prone to nocturnal polyuria. Superimposed on age-related changes in bladder function and mobility, altered ADH regulation can contribute to urinary incontinence. Older patients with nocturnal polyuria may benefit from administration of the ADH analog desmopressin (DDAVP), in addition to other interventions such as detrusor muscle relaxants and behavioral interventions.
See Chapter 88 for additional information on effects of aging on salt and water regulation.
Hypothalamic–Pituitary–Adrenal Axis
Compared to the other hypothalamic–pituitary–end-organ axes, hypothalamic–pituitary–glucocorticoid function is relatively intact with aging in humans (Table 107-3). The cortisol secretion rate is decreased with aging, but this is matched by a decrease in the cortisol metabolic clearance rate. As a result, basal plasma concentrations of cortisol are unchanged with aging even in very old subjects. Consistent with intact cortisol secretion, basal ACTH levels are also unaffected by aging. Furthermore, glucocorticoid responsiveness of the HPA axis to stimulation is well-maintained in older adults, with intact cortisol responses to exogenous ACTH stimulation (except at very low doses of ACTH [0.06 μg]) and normal or slightly prolonged cortisol and ACTH responses to metyrapone, ovine CRH, and insulin-induced hypoglycemia. ACTH pulse frequency appears to be maintained in older men, suggesting intact hypothalamic regulation of glucocorticoid function. Moreover, the circadian rhythm of ACTH and cortisol secretion is intact in healthy older subjects although the amplitude of the cortisol rhythm is reduced and the nighttime cortisol nadir is increased in older compared to young adults. In addition, a phase advance of the cortisol rhythm occurs in older people, with earlier nadir and peak secretion, as compared to young adults. This phase advance has been attributed to behavioral changes, possibly reflecting an earlier bedtime in older adults.
ACTH levels | ↔ |
ACTH response to CRH stimulation | ↔ |
Cortisol production | ↓ |
Cortisol clearance from plasma | ↓ |
Plasma cortisol levels | ↔ |
Cortisol response to stimulation | |
ACTH | ↔ |
Metyrapone, hypoglycemia | ↔/↑ |
Surgical stress | ↑ |
HPA axis sensitivity to glucocorticoid feedback | ↓ |
Although still controversial, many studies have reported age-related alterations in the cortisol response to stress. After a stressful stimulus such as surgery, peak cortisol levels are higher and remain elevated longer in older compared to young adult subjects. Furthermore, dexamethasone is less effective in suppressing cortisol levels in older subjects, and in studies using metyrapone to remove endogenous cortisol feedback inhibition, the decline in ACTH levels after exogenous cortisol infusion was blunted and delayed in older compared to young adult subjects. Taken together, these studies indicate that the sensitivity of the HPA axis to glucocorticoid negative feedback is decreased with aging. The clinical implications of this decreased responsiveness to glucocorticoid feedback inhibition are unclear. However, it has been proposed that the resulting chronic increase in glucocorticoid exposure may damage hippocampal neurons regulating glucocorticoid secretion and important to cognitive function, leading to a vicious cycle of further glucocorticoid hypersecretion and damage to mechanisms regulating HPA feedback inhibition.
Although glucocorticoid function is well-preserved with aging, certain factors may interfere with testing of HPA axis function in older patients (Table 107-4
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree