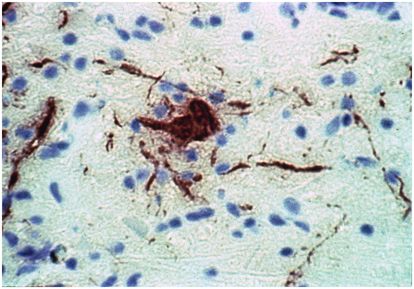
Figure 76.1 Mononuclear inflammatory cells accumulate around blood vessel in a fatal case of Japanese encephalitis. These perivascular “cuffs” are a characteristic histopathologic finding in the central nervous system (CNS) during acute viral encephalitis. Hematoxylin & eosin, 100×. (Courtesy of Richard T. Johnson, MD, Department of Neurology, The Johns Hopkins University School of Medicine.)
Etiology
More than 100 different viruses can infect the human CNS, but a much smaller number cause the vast majority of viral encephalitis cases. The most relevant pathogens come from the following viral families: Herpesviridae, Picornaviridae, Retroviridae, Paramyxoviridae, and arthropod-borne RNA viruses including the Togaviridae, Flaviviridae, Bunyaviridae, and Reoviridae families (Table 76.1). The acquired immunodeficiency syndrome (AIDS) epidemic, the therapeutic use of immunosuppression in transplant recipients or patients with autoimmune disease, and the immune defects that occur in oncology patients as a by-product of chemotherapy have all resulted in the identification of new infectious disease processes that can cause signs and symptoms consistent with acute encephalitis. Many immunocompromised hosts now require discrimination between an ever-expanding list of potential infectious and noninfectious causes of an acute encephalitis-like clinical picture (Table 76.2).
| Herpesviridae Herpes simplex virus Varicella-zoster virus Cytomegalovirus Epstein–Barr virus Human herpesvirus 6 B virus Bunyaviridae California serogroup viruses La Crosse virus Jamestown Canyon virus Snowshoe hare virus Togaviridae (alphaviruses) Eastern equine encephalitis virus Western equine encephalitis virus Venezuelan equine encephalitis virus Flaviviridae Japanese encephalitis virus St. Louis encephalitis virus West Nile virus Tick-borne encephalitis virus Dengue fever encephalitis virus | Reoviridae Colorado tick fever virus Picornaviridae Echovirus Coxsackievirus Poliovirus Enterovirus 71 Retroviridae Human immunodeficiency virus, type 1 Papovaviridae JC virus Orthomyxoviridae Influenza virus Paramyxoviridae Measles virus Mumps virus Nipah virus Hendra virus Miscellaneous viruses Adenovirus Lymphocytic choriomeningitis virus Rabies virus |
| Infectious | Noninfectious |
|---|---|
| Bacterial Acute bacterial meningitis Brain abscess Parameningeal infection Subdural empyema Venous sinus thrombophlebitis CNS Lyme disease Neurosyphilis Whipple’s disease Bacterial toxin-mediated process Fungal Fungal meningitis Fungal brain abscess Parasitic Toxoplasma gondii abscess Cerebral malaria Human African trypanosomiasis Amebic Naegleria fowleri meningoencephalitis Acanthamoeba meningoencephalitis | Parainfectious/autoimmune Reye’s syndrome Postinfectious encephalomyelitis Postvaccination encephalomyelitis Neoplastic Primary or metastatic brain tumor Paraneoplastic disorder Neoplastic meningitis Cerebrovascular Acute ischemic stroke Subdural hematoma CNS vasculitis Systemic Metabolic encephalopathy Connective tissue disease Drug intoxication Epileptic Seizures/postictal state Traumatic Acute head injury |
Epidemiology
The viruses that cause acute encephalitis vary widely in their epidemiology. In many cases, the identification of a particular causative agent can be aided by clues derived from the surrounding environment (geography, season) as well as from a careful review of the patient’s background (sexual behavior, intravenous drug use, travel, occupation, arthropod or animal contacts, vaccine history, and exposure to ill persons). One important point is that many viruses causing acute encephalitis are transmitted to humans via infected mosquitos or ticks and thus produce disease in the summer or early fall months when the vectors are prevalent. The more common viral encephalitides have notable epidemiologic features associated with them (Table 76.3).
| Family/virus | Affected hosts | Peak season/pattern | Geography/incidence | Clinical presentation | Epidemiologic clues |
|---|---|---|---|---|---|
| Herpesviridae | |||||
| HSV | All ages | Year-round; endemic | Ubiquitous (~2500 cases/yr) | Focal neurologic deficits; seizures; bizarre behavior | |
| VZV | Healthy and immunocompromised adults; infants | Year-round; endemic | Ubiquitous | Ataxia; stroke-like episodes; can have an accompanying myelitis | Recent primary varicella rash or herpes zoster dermatomal rash |
| CMV | Immunocompromised adults; infants | Year-round; endemic | Ubiquitous | Periventricular lesions on brain MRI; accompanying lumbosacral polyradiculitis | Known HIV+ individuals; post-transplant recipients (especially bone marrow recipients) |
| Retroviridae | |||||
| HIV | All ages | Year-round; endemic | Ubiquitous (3000–4000 cases/y) | Subacute cognitive deficits; psychomotor slowing | High-risk sexual practices; intravenous drug use |
| Papovaviridae | |||||
| JC virus | Immunocompromised adults | Year-round; endemic | Ubiquitous (400–800 cases/y) | Focal neurologic deficits; multifocal MRI lesions | HIV+ individuals; post-transplantion or immunotherapy |
| Togaviridae | |||||
| Eastern equine encephalitis virus | Young and elderly | Summer and fall; endemic/sporadic | East and Gulf Coasts (5–10 cases/y) | Fulminant deficits; seizures; coma | Outdoor occupation or activities; proximity to marshes or standing water |
| Western equine encephalitis virus | Young and elderly | Summer and fall; endemic/sporadic | Midwest and Western States (10–15 cases/y) | Nonfocal deficits; headache | Outdoor occupation or activities; travel or habitation in rural areas |
| Flaviviridae | |||||
| West Nile virus | All ages (but most cases in the young and the elderly) | Summer and fall; epidemic | Nationwide (2000–4000 cases/y over the last few years) | Nonfocal deficits; headache; ~20% with a poliomyelitis-like illness | Outdoor exposure (urban or rural); most cases are concentrated in a few states each season |
| St. Louis encephalitis virus | Young and elderly | Summer and fall; epidemic | Nationwide (~100 cases/y; range 2–1967 cases/y) | Nonfocal deficits; headache | Outdoor exposure; endemic in rural areas in the West; sporadic urban outbreaks in the Eastern States |
| Bunyaviridae | |||||
| La Crosse virus | Young | Summer and fall; endemic and small case clusters | Midwest and Eastern States (75–100 cases/y) | Often asymptomatic; can cause seizures | Outdoor activities; suburban cases occur near wooded areas |
| Picornaviridae | |||||
| Echoviruses Coxsackieviruses Polioviruses Unclassified viruses (EV-68–EV-71) | Young, especially agammaglobulinemic children | Summer and fall; epidemic | Nationwide (~1000 cases/y) | Accompanying viral exanthem, conjunctivitis, myopericarditis, herpangina, hand-foot-and-mouth disease | Known community epidemic of picornavirus |
| Rhabdoviridae | |||||
| Rabies | All ages | Year-round; endemic | Nationwide (10–15 cases/y) | Prior animal bite or scratch; autonomic symptoms in ~80%; paralysis in ~20% | Animal contact |
Abbreviations: HIV = human immunodeficiency virus; CMV = cytomegalovirus; MRI = magnetic resonance imaging; HSV = herpes simplex virus; VZV = varicella-zoster virus.
Pathogenesis
Most viruses gain entry into the CNS either through hematogenous or intraneural spread. Bunyaviridae, Flaviviridae, and Togaviridae seed the CNS from the bloodstream after subcutaneous inoculation by the insect vector and replication in local tissues. Other neurotropic viruses enter the host via the respiratory tract (e.g., adenovirus, measles, influenza) or the gastrointestinal tract (e.g., enteroviruses). Rabies virus reaches the CNS via intra-axonal transport in sensory nerves that innervate the skin. The pathogenesis of herpes simplex virus (HSV) encephalitis remains incompletely understood, but virus passage along the olfactory and trigeminal nerve tracts from ganglia where it can reactivate from latency likely explains the classic temporal lobe localization. Host factors play a role in both the susceptibility to and the severity of viral encephalitis. Chronic enteroviral meningoencephalitis occurs mostly in patients with agammaglobulinemia, whereas acute measles, cytomegalovirus (CMV), and varicella-zoster virus (VZV) encephalitis usually occurs in patients with impaired cellular immunity.
Once inside the CNS, encephalitic viruses cause pathology either due to a direct cytopathic effect or as a result of immune-mediated injury. The tropism of these pathogens for various parenchymal cell populations varies during acute encephalitis, but those directly infecting neurons often cause particularly severe disease (Figure 76.2). The ensuing histopathologic changes reflecting the host response include the perivascular infiltration of mononuclear inflammatory cells (Figure 76.1), a reactive astrocytosis, the formation of glial nodules, and neuronophagia. Cytotoxic T cells and phagocytic macrophages may actually be the effectors of much of the resulting neural injury. It is also likely that soluble immune factors (cytokines, chemokines, nitric oxide, etc.) contribute to disease pathogenesis in complex ways, both to the benefit and the detriment of the infected host. While factors such as the interferons (α, β, and γ) and their regulatory transacting proteins may act to limit CNS virus replication, others such as interleukin (IL)-1β, IL-6, and tumor necrosis factor (TNF)-α may have injurious properties in humans and clearly make viral encephalitis worse in animal models of these diseases.