Variable
Patients with AKI (n = 15)
Patients without AKI (n = 21)
P value
Age in years
63.8 ± 7.88
67.4 ± 7.18
0.87
Gender (male (%))
11 (73)
6 (29)
0.20
Diabetes (%)
11 (73)
15 (7)
0.76
Baseline sCr (mg/dL)
1.69 ± 0.44
1.25 ± 0.35
0.10
sCr at 36 h post-op (mg/dL)
2.26 ± 0.54
1.43 ± 0.36
0.04
Baseline eGFR (ml/min/1.73 m2)
53 ± 15
74 ± 18
0.08
Left ventricular ejection fraction <40 % (%)
5 (33)
8 (38)
0.91
Peripheral vascular disease (%)
3 (20)
4 (19)
0.89
Baseline WBC (cells/mm3)
8.7 ± 3.1
7.66 ± 2.6
0.54
Surgery type:
CABG (%)
Valve only (%)
Combined (%)
7 (47)
5 (33)
3 (20)
12 (57)
7 (33)
2 (10)
0.56
CPB time (min)
118 ± 33
103 ± 29
0.64
Cross clamp time (min)
80 ± 12
70 ± 9
0.10
Use of pressors for >24 h post-operatively (%)
5 (33)
5 (24)
0.21
CCF risk score
6.9 ± 2.1
4.87 ± 1.9
0.06
Need for post-operative dialysis (%)
3 (20)
0
0.07
In-hospital death (%)
2 (13)
1 (5)
0.4
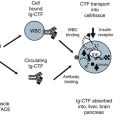
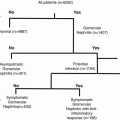
A preoperative risk assessment for the occurrence of AKI was performed using both the renal risk stratification system of Chertow as well as the Cleveland Clinic Foundation (CCF) scoring system (excludes systemic infection) (Thakar et al. 2005; Chertow et al. 1997). Based upon these scoring system which utilize clinical variables and baseline renal function, there was no statistically significant difference in baseline risk between those patients who developed AKI and those who did not. However, there was a trend toward higher CCF scores in the group that developed AKI (see Table 8.1). Most patients who developed AKI had white blood cells in urine (n = 13) indicating an innate immune mediated inflammatory reaction. Patients who did not develop AKI did not have WBC in their urine.
Exclusion criteria included: age <18 years, failure to obtain consent, recent (within 3 month) development of AKI (defined as a rise in serum creatinine greater than 0.3 mg/dL from a stable baseline creatinine if this data was available), recent infectious disease (defined as a documented fever >38 °C within 1 week of surgery, use of antibiotics within 7 days of surgery with the exclusion of pre-operative or intra-operative prophylactic antibiotics), or a white blood cell count (WBC) >10,000/mm3, end-stage renal disease, baseline serum creatinine >2.0 mg/dL, use of intra-aortic balloon pump, pre-operative use of inotropic agents or vasopressors, pre-operative cardiogenic shock, post-operative anuria and pre-operative use of intravenous contrast within 7 days of the surgery. At total of 78 patients were screened and 36 enrolled. Post-operatively all patient physiological parameters (blood pressure, pulse, temperature, urine output, use of inotropes/vasopressors) were recorded.
8.2.2 Sample Collection
Time matched blood and urine specimens were collected immediately pre-operatively, and then at 1–3 h, 5–7 h and 9–11 h on the day of surgery and every day thereafter out to 6 days post-operatively. Blood was analyzed for creatinine, electrolytes, white blood cell count, hemoglobin and biomarkers as described below. Duplicate urine and blood specimens were stored at −70 °C until analyzed for biomarkers. A protease inhibitor cocktail (contains 0.1 mg/mL Pefabloc SC® (Centerchem Inc, Norwalk, CT), 0.1 mg/mL leupeptin (SigmaAldrich, St. Louis MO), and 1 mM sodium azide (SigmaAldrich, St. Louis MO)) was added to each urine sample. Ejection fraction was estimated visually by surgeon. Surgical time for the bypass (min) and urinary output (ml/h) were recorded. Granular casts were measured on urine sample at time of collection by spinning the urine and viewing under high power light microscopy. Serum creatinine values and a complete white blood cell count (WBC) was obtained using specimens sent to the hospital laboratory. Duplicate specimens were stored at −70 °C until analyzer for remaining biomarkers.
8.2.3 Biomarker Analysis
Quantitative urinalysis for albumin and creatinine were measured using the DCA Vantage instrument (Siemens Healthcare Diagnostics, Tarrytown, New York). Urinalysis strip results for occult blood, creatinine, protein, albumin, nitrite, ketone, glucose, pH and luekocytes were measured using the CLINITEK Status instrument (Siemens Healthcare Diagnostics, Tarrytown, New York) with the MULTISTIX PRO® 10LS and CLINITEK Microalbumin®. Casts are determined by spinning the urine and viewing under high power light microscopy
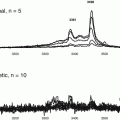
Enzyme-linked immunosorbent assay (ELISA) measurements were made with commercially available kits. Urine and serum IL-6 used kits from E-Bioscience, San Diego, CA (Cat No. 88–706). Control and standards were preformed according to manufacturer’s instructions. Serum measurement of Cystatin C used kits from Biovendor Laboratory Medicine Inc, Czech Republic (Cat No RD191009100). Serum measurement of Adpn used kits from Panomics Inc, Fremont, CA (Cat No K1001-1). Urine measurement of NGAL used kits from Affinity BioReagents Inc, Colden, CO (Cat No 037). All kits used monoclonal antibodies directed against the human peptide in a sandwich assay format with enzyme labels. Urine assays for Uristatin were performed by Brendan Bioscience (Hopewell MA) following literature competitive antibody ELISA method (Pugia et al. 2007b) (see Chap. 8 and Supplement Materials). Control and standards were preformed according to manufactures instructions.
Urine assays for ABP-26 was performed by Brendan Bioscience (Hopewell, MA) following the literature ELISA Nephroscreen method (Thompson et al. 1985). Briefly, ELISA plates (Corning®, 9018) were coated overnight at 4–8 °C with anti- ABP-26 (Uro 4 antibody, 8040–99, Covance) in bicarbonate buffer, pH 9.6 at 8 ug/ml. The plates were washed 2X with coating buffer then blocked with bicarbonate buffer containing 0.2 % bovine serum albumin (Sigma, A8022) for 2 h at 4–8 °C. The block was decanted and the plates were stabilized with 10 % sucrose for 1 h at room temperature (RT). The stabilizer was decanted and the plates were dried in vaco then sealed in a mylar pouch with desiccant. The detection conjugate was made by conjugating a second anti-ABP26 antibody (Uro-4a, 8045–99, Covance Inc) to HRP (Uro4a-HRP). The standard curve was constructed by diluting ABP26 (R&D Systems, 1180-SE) from 500 to 7.81 ng/ml in wash/sample diluent (W/SD) prior to adding it to the plate along with the samples which were diluted 1:100 in W/SD.
Urine samples and standards were diluted and 100 μL was added to each well and incubated for 4 h at RT. The fluid was decanted and the plate was washed 3X. The Uro4a-HRP was added to each well and incubated for 2 h at RT. The conjugate was decanted and the plate was washed 3X. The OPD substrate (Sigma, P-8412, 4 mg/ml in 100 mM citrate, pH 5.0) was added and incubated at RT for 20–30 min. The reaction was stopped using 3 N H2SO4 and the absorbance was read at A492. A linear regression was performed using the data from the standard curve. The concentration of ABP-26 was calculated for each sample using the regression values derived from the standard curve.
8.2.4 Statistical Analysis
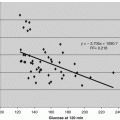
AKI was defined as a rise in serum creatinine >0.3 mg/dL occurring within 48 h after CPB surgery and sustained for >48 h. Patients were parsed into two groups (AKI v. no AKI) based upon meeting this criteria of AKI. SAS version 8.2 was used for analyses. Values for groups are shown in Tables 8.2 and 8.3. To compare continuous variables, we used a two-sample t test or Mann–Whitney rank sum test, and to compare categorical variables we used the χ 2 or Fisher’s exact test, as indicated. To measure the sensitivity and specificity for urine uristatin and IL-6 at different cutoff values, a conventional receiver-operating characteristic (ROC) curve was generated for preoperative uristatin and IL-6. We calculated the area under the curve to ascertain the quality of uristatin as a biomarker. An area of 0.5 is no better than expected by chance, whereas a value of 1.0 signifies a perfect biomarker. Univariate and multivariate stepwise multiple logistic regression analyses were undertaken to assess predictors of acute kidney injury. Potential independent predictor variables included age, sex, ethnic origin, cardiopulmonary bypass time, ejection fraction, urine albumin/creatinine, urine and serum IL-6, and urine uristatin. We judged p < 0 · 05 significant
Table 8.2
Summary statistics for the demographic and clinical variables
Clinical variables | ARF | n | Mean | SD | SE | Median | GM | Min | Max | Q25 | Q75 |
|---|---|---|---|---|---|---|---|---|---|---|---|
Age | 0 | 22 | 63.82 | 7.88 | 1.68 | 64.00 | 63.36 | 51.00 | 79.00 | 58.25 | 67.00 |
1 | 15 | 67.40 | 7.18 | 1.85 | 68.00 | 67.04 | 56.00 | 78.00 | 63.50 | 72.50 | |
Ejection fraction | 0 | 22 | 40.45 | 8.99 | 1.92 | 37.50 | 39.50 | 25.00 | 55.00 | 35.00 | 45.00 |
1 | 15 | 28.67 | 11.41 | 2.95 | 25.00 | 26.89 | 15.00 | 55.00 | 20.00 | 30.00 | |
Urine bypass time | 0 | 22 | 70.05 | 8.38 | 1.79 | 68.50 | 69.60 | 60.00 | 94.00 | 65.00 | 73.50 |
1 | 15 | 80.13 | 12.08 | 3.12 | 78.00 | 79.27 | 60.00 | 98.00 | 72.00 | 89.50 | |
Urine output | 0 | 22 | 96.36 | 23.96 | 5.11 | 95.00 | 93.62 | 60.00 | 150.00 | 80.00 | 118.75 |
1 | 15 | 87.33 | 32.94 | 8.51 | 80.00 | 76.94 | 10.00 | 125.00 | 75.00 | 120.00 | |
WBC | 0 | 22 | 7.66 | 2.61 | 0.56 | 7.35 | 7.25 | 4.30 | 13.40 | 5.48 | 8.98 |
1 | 15 | 8.69 | 3.07 | 0.79 | 8.00 | 8.27 | 5.60 | 16.80 | 6.55 | 9.65 |
Table 8.3
Summary statistics for the mean of the patient’s observations up to and including the time point when the patient was determine to be ARF
Response | ARF | n | Mean | SD | SE | Median | GM | Min
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|
|---|