Our understanding of the molecular genetics of acute leukemias has improved dramatically over the past decade. Fueled in part by the availability of the complete sequence of the human genome, more than 100 different mutations have been identified that can be causally implicated in the pathogenesis of acute leukemias. At first glance, the plethora of mutations presents a discouraging prospect for the development of molecular targeted therapies. However, far more mutations are identified than there are phenotypes of acute leukemia, and a theme is developed in this chapter that many of these mutations must target similar signal transduction or transcriptional pathways. Thus, it is plausible to consider therapeutic approaches that target these shared pathways of transformation. Although many mutations remain to be identified, those observed thus far have provided critical insights into the pathophysiology of leukemia and the development of novel therapeutic targets.
LEUKEMIC STEM CELL
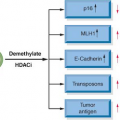
An important emerging concept in the pathobiology of leukemia is the existence of a “leukemic stem cell.” In normal hematopoietic development, there is a rare population of hematopoietic stem cells that have self-renewal capacity and give rise to multipotent hematopoietic progenitors. These multipotent myeloid or lymphoid progenitors do not have self-renewal capacity but mature into normal terminally differentiated cells in the peripheral blood. It is hypothesized that there is a leukemic stem cell that has limitless self-renewal capacity and gives rise to clonogenic leukemic progenitors that do not have self-renewal capacity but are incapable of normal hematopoietic differentiation.
The first convincing evidence in support of the existence of a leukemic stem cell was derived from experiments in which human leukemic cells were injected into immunodeficient NOD-SCID mice (nonobese diabetic mice with severe combined immunodeficiency disease).
1 These data show that the resultant leukemias are derived from as few as 1:1,000 to 1:10,000 cells, indicating that there is a rare population of human leukemic cells that have self-renewal capacity in this assay. These cells have similar immunophenotypes to normal self-renewing hematopoietic progenitors and suggest that the leukemogenic mutation occurs in a hematopoietic stem cell. In support of this hypothesis, clonal cytogenetic abnormalities, such as the t(9;22), have been detected in primitive hematopoietic progenitors such as CD34+/CD38− cells (reviewed in ref. 2).
However, this paradigm has been recently challenged and revised. First, new protocols that enhance engraftment of human leukemia in xenotransplant setting have been described suggesting the importance of homing and the role of microenvironment.
3,
4 Data also show that it may be the leukemic oncogenes themselves that confer properties of self-renewal. In a murine system, transduction of the leukemia oncogenes
MLL-ENL (mixed-lineage leukemia 1-eleven nineteen leukemia),
MOZ-TIF2 (monocytic leukemia zinc finger protein-transcriptional intermediary factor 2), or
MLL-AF9 (mixed-lineage leukemia
ALL1-fused gene from chromosome 9 protein) can confer properties of self-renewal to purified committed hematopoietic progenitors that have no capacity for self-renewal.
5,
6 MLL-AF9 oncogene-induced leukemia showed that leukemic stem cells account for as high as 25% of the leukemic cells while exhibiting mature myeloid immunophenotype.
7 High frequency of leukemic stem cells have been also described in a murine AML
Sfpi−/−.
8 HOX family and
Notch genes, frequent mutational targets in acute leukemia, as discussed later in this chapter, are being found to have significant roles in hematopoietic stem cell self-renewal.
9 Secondary mutations may also enable activation of similar self-renewal pathways. For example, analysis of cells from acute myeloid leukemia (AML) blast crisis in chronic myeloid leukemia (CML) shows a shift in the leukemic stem cell to an immunophenotype of a committed myeloid progenitor and concurrent nuclear localization of
β-catenin, a process thought to increase stem cell self-renewal.
10 Further investigation will be required to determine to what extent these findings can be extrapolated to other
leukemia oncogenes and to human leukemia. One of the major goals is identification of transcriptional programs, genes, and pathways that confer limitless self-renewal and may be targets for therapeutic intervention. In addition, these transcriptional programs may be commandeered to confer properties of self-renewal to adult somatic tissues for therapeutic purposes such as tissue regeneration. For further development of this issue, see
Chapter 11.
ELUCIDATION OF GENETIC EVENTS IN ACUTE LEUKEMIA
The search for causative mutations in acute leukemia has accelerated in recent years because of the availability of new means for evaluating genome integrity in leukemic cells. The bulk of known translocations and deletions was found by analyzing conventionally stained chromosomal banding patterns of karyotypes. Classic karyotypic analysis is able to identify lesions with a resolution of 5 to 10 Mb. An improvement on this technique is spectral karyotyping, in which 24 unique dye combinations are used on conjugated probes specific to each chromosome. Mutations can be observed in the resolution of 1 to 2 Mb. This technique is particularly useful in finding otherwise cryptic or complex translocations as chromatin in derivative chromosomes are “painted” to identify their chromosome of origin.
New technologies such as comparative genomic hybridization (CGH) and single nucleotide polymorphism (SNP) arrays allow detailed (<35 kb) mapping of unbalanced insertions or deletions (reviewed in refs. 11 and 12). Array comparative genomic hybridization determines DNA copy gain or loss by comparing the hybridization of sample DNA to a series of clones or oligonucleotides from regions throughout the genome bound to a chip with a normal reference DNA sample. SNP arrays differ in that oligomers of SNP sequences representing known alleles throughout the genome are used in the array to measure changes in expected genotype and copy number. Finally, low-cost, high-throughput sequencing and microarray-based resequencing techniques have allowed identification of new somatic mutations at the single nucleotide level and are making enormous inroads into the pathogenesis, prognosis, and classification of leukemias with “normal” cytogenetics. Sequencing of complete AML genomes has been accomplished and alternative strategies limiting sequencing to the transcriptome or kinase-encoding regions (kinome) to reduce cost and effort are also uncovering new, significant mutations.
13,
14,
15 Worldwide initiatives to sequence large numbers of cancer genomes, including leukemia subtypes, are being coordinated and cataloged through the International Cancer Genome Consortium (http://www.icgc.org).
16
RECURRING CHROMOSOMAL ABNORMALITIES IN ACUTE LEUKEMIA
Nonrandom chromosomal abnormalities can be detected in the majority of cases of acute leukemia using classic high-resolution banding techniques. These include balanced reciprocal chromosomal translocations, such as t(8;21) (q22;q22) or t(15;17)(q22;q21); internal deletions of single chromosomes, such as 5q- or 7q-; gain or loss of whole chromosomes (+8 or −7); or chromosome inversions, such as inv(3), inv(16), or inv(8). Complex chromosomal abnormalities are observed in approximately 15% of
de novo cases that do not have an antecedent hematologic disorder; this constitutes a clinical group of patients with particularly poor prognoses. Selected recurring cytogenetic abnormalities observed in acute leukemias are annotated in
Table 31.1.
Initial insights into the pathobiology of acute leukemias were derived from analysis and molecular cloning of recurring chromosomal translocations.
17 First, it appears that certain genomic loci are associated with specific subtypes of leukemia. For example, more than 40 different recurring translocations target the
MLL gene locus on chromosome 11q23 and are generally associated with a myelomonocytic or monocytic AML phenotype (FAB M4 or M5). As another example, five different translocations target the retinoic acid receptor alpha locus (RAR-
α), including the t(15;17)(q22;q21), which is the most common of these, and are all associated with an acute promyelocytic leukemia (APL) phenotype (FAB M3). Second, it appears that most chromosomal translocations associated with AMLs target transcription factors that are important for normal hematopoietic development. For example, more than a dozen translocations target the core-binding factor (CBF), a heterodimeric transcriptional complex essential for hematopoiesis. The translocations that target
CBF, such as the t(8;21), result in expression of dominant negative inhibitors of normal CBF function, such as the AML1/ETO fusion. Thus, one consequence of many of these chromosomal translocations in acute leukemias is impaired hematopoietic differentiation. A third general observation has been that fusion genes associated with acute leukemias are necessary but not sufficient to cause acute leukemia.
CHROMOSOMAL TRANSLOCATIONS THAT TARGET CORE-BINDING FACTOR
CBF is targeted by more than a dozen different chromosomal translocations in acute leukemias, including the t(8;21) or inv(16), observed in approximately 20% of AMLs, and the t(12;21), present in approximately 25% of patients with pediatric B-cell acute lymphoblastic leukemia (ALL).
18 Adult patients with CBF leukemias have a favorable prognosis and the TEL/AML1 fusion that is expressed as a consequence of t(12;21) in children confers a favorable prognosis among B-cell ALL.
19
CBF is a heterodimeric transcription factor composed of the AML1 and CBF
β proteins that is critical for normal hematopoietic development. Loss of function of either subunit results in a complete lack of definitive hematopoiesis.
20,
21 The AML1 subunit of CBF contacts DNA but only weakly transactivates target genes as a monomer. When bound to its heterodimeric partner CBF
β, which does not contact DNA itself, transactivation of CBF target genes is dramatically enhanced.
19 CBF transactivates a spectrum of target genes that are important in normal myeloid development, including cytokines (e.g., granulocyte-macrophage colony-stimulating factor) and cytokine receptors (such as M-CSF receptor), as well as in lymphoid development, such as the TCR
β enhancer and the immunoglobulin heavy-chain loci. Because CBF targets genes that are important for normal hematopoietic development, a mutation or gene rearrangement that resulted in loss of function of either
AML1 or
CBFβ might be expected to impair hematopoietic differentiation.
19Compelling evidence has been shown that translocations that target CBF result in loss of function through dominant negative inhibition. The
AML1/ETO fusion associated with t(8;21) and the CBF
β/SMMHC (smooth muscle myosin heavy chain) fusion associated with inv(16) are dominant negative inhibitors of CBF and impair hematopoietic differentiation. Expression of either the
AML1/ETO or
CBFβ/SMMHCC fusion genes from their endogenous promoter in mice completely inhibits the function of the residual AML1 or CBF
β alleles, resulting in a lack of definitive hematopoiesis and resultant embryonic lethality.
20,
21 The phenotype observed is the same as that seen in
AML1−/− or
CBFβ−/− mice, indicating that the AML1/ETO or CBF
β/SMMHC fusions, respectively, act as complete dominant negative inhibitors of the native proteins. Repression of CBF target genes by the AML1/ETO or CBF
β/SMMHC fusions is mediated by aberrant recruitment of the nuclear corepressor complex, as it is for the PML/RAR-
α fusion (reviewed in refs. 22 and 23). Thus, it has been suggested that histone deacetylase, a component of the corepressor complex, may be a therapeutic target for leukemias associated with translocations that target CBF.
24Although expression of AML1/ETO leads to alterations of gene expression and hematopoietic cell proliferation leukemia and confers the ability to serially replate in methylcellulose culture (a measure of self-renewal potential), this does not result in development of leukemia in an animal model. However coexpression of an alternatively spliced isoform of the
AML1-ETO transcript,
AML1-ETO9a, that includes an extra exon, exon 9a, of the
ETO gene (
AML1-ETO9a encodes a C terminally truncated AML1-ETO protein of 575 amino acids) leads to a rapid development of leukemia in a mouse retroviral transduction-transplantation model.
25 The presence of
AML1-ETO9a closely correlates with presence of activating c-Kit mutations in humans, conferring a poor prognosis.
26 Similarly, expression of CBF
β/SMMHC in adult hematopoietic cells results in leukemia only after a markedly prolonged latency; this latency can be shortened using mutagenesis strategies.
27 Translocations that target
CBF impair hematopoietic differentiation and confer certain properties of leukemic stem cells, such as the ability to serially replate, but are not sufficient to cause leukemia. In some situations fusion proteins from alternatively spliced isoforms of a chromosomal translocation may work together to induce cancer development.
26
CHROMOSOMAL TRANSLOCATIONS THAT TARGET THE RETINOIC ACID RECEPTOR ALPHA GENE
The empiric observation that all-
trans-retinoic acid (ATRA) induces complete responses in patients with APL drove the subsequent cloning of the t(15;17)(q22;q21) fusion gene involving the RAR-
α locus. Several groups demonstrated at approximately the same time that the RAR-
α (
RARα) gene on chromosome 17 was fused to a novel partner that was eventually identified as the promyelocytic leukemia (PML) gene.
28,
29,
30 Two reciprocal fusion RNA species are produced as a consequence of the translocation,
RARα/PML and
PML/RARα. The PML/RAR-
α fusion protein contains the zinc finger of PML fused to the DNA- and protein-binding domains of RAR-
α. Several other chromosomal translocations associated with an APL phenotype have been cloned and characterized. Each of these targets the
RARα locus, with fusion to various partners (see
Table 31.1). The best studied of these is the PLZF/RAR-
α fusion, which also aberrantly recruits the nuclear corepressor complex (
Table 31.1). However, in contrast with the PML/RAR-
α fusion, ATRA is not able to relieve corepression mediated by the PLZF/RAR-
α fusion, and thus is not effective in patients who harbor the t(11;17) associated with this fusion gene.
31As with CBF fusions, the PML/RAR-
α fusion protein functions as a dominant inhibitory oncogene for RAR-
α-interacting proteins, including RXR-
α. In addition, the PML/RAR-
α fusion interferes with the function of the native PML protein, which is thought to function as a tumor suppressor gene.
32 Collectively, the dominant interfering activities of the PML/RAR-
α fusion protein result in a block in differentiation at the promyelocyte stage of development. The clinical response of these patients to ATRA is explained by the ability of this retinoid to bind to the PML/RAR-
α fusion protein and reverse repression of target genes required for normal hematopoietic development. The ability of the PML/RAR-
α fusion protein to repress transcription is partly due to the aberrant recruitment of the nuclear corepressor complex, including histone deacetylase, suggesting that pharmacologic agents that inhibit histone deacetylases may be useful in therapy of APL.
24,
33The transforming properties of the
PML/RARα fusion gene have been tested in murine models. Expression of
PML/RAR-α in transgenic mice from promoters that direct expression to the promyelocyte compartment result in an APL-like phenotype.
34,
35,
36 However, there is approximately a 6-month lag before the development of leukemia, incomplete penetrance of approximately 15% to 30%, and acquired karyotypic abnormalities, all suggesting that second mutations are required for induction of leukemia. In at least some cases, activating mutations in
FLT3, as discussed later in “Activating Mutations in
FLT3 and
KIT,” may be the additional mutation required. ATRA is efficacious in leukemic animals, expressing both PML/RAR-
α and activated FLT3, and this model has allowed for the preclinical testing of novel agents such as arsenic trioxide.
37
CHROMOSOMAL TRANSLOCATIONS THAT TARGET HOX FAMILY MEMBERS
The large HOX family of transcription factors is important in patterning in vertebrate development and also plays a critical role in normal hematopoietic development (reviewed in
ref. 38).
HOX genes may also be targeted by chromosomal translocations, with examples including the NUP98/HOXA9 and NUP98/HOXD13 fusions, associated with t(7;11) and t(2;11), respectively (see
Table 31.1).
39,
40 HOX gene expression is tightly regulated during hematopoietic development. HOXA9, for example, is expressed in early hematopoietic progenitor cells but is down-regulated during hematopoietic differentiation and is undetectable in terminally differentiated cells. It has been suggested that unregulated overexpression of the HOXA9 moiety from the constitutively active NUP98 promoter may result in aberrant differentiation. Experimental support for this hypothesis includes the observation that the NUP98/HOXA9 fusion protein can transform 3T3 fibroblasts, an activity that requires the HOXA9 DNA-binding domain.
41The contribution of the NUP98 moiety to leukemic transformation is not fully understood. NUP98 is normally a component of the nuclear pore complex and is constitutively and ubiquitously expressed. However, several lines of evidence suggest that NUP98 contributes more than a constitutively activated promoter. For example, NUP98 motifs known as
FG repeats are essential for transformation and may serve to recruit transcriptional coactivators, such as CBP/p300, to HOXA9 DNA-binding sites.
41 In murine models of leukemia, overexpression of HOXA9 alone is not sufficient to cause AML, but coexpression of HOXA9 with transcriptional cofactors, such as MEIS1, results in efficient induction of AML. Thus, the NUP98 moiety in the context of the NUP98/HOXA9 fusion may serve multiple functions, including provision of an active promoter, and recruitment of transcriptional coactivators such as CBP/p300 that subserve the function of other cofactors such as MEIS1. Epidemiologic evidence that the NUP98 moiety contributes to leukemogenesis includes the observation that there are now a spectrum of fusion proteins involving components of the nuclear pore that are targeted by chromosomal translocations in acute leukemias. These include
NUP98 and
NUP214 fused to a diverse group of partners, including
HOXA9 and
HOXD13, and the
DDX10, PMX1, DEK, and
ABL1 genes, respectively.
It has been hypothesized that dysregulated
HOX gene expression may be important in leukemias that do not directly target HOX family members. Several proteins that are upstream of HOX expression have been observed as fusion genes associated with AML, the most frequent of these are
MLL gene rearrangements. More than 40 chromosomal translocations target
MLL and result in fusions of
MLL with a broad spectrum of partners. However, a common biologic feature of all of these may be their ability to dysregulate
HOX gene expression during hematopoietic development. For example, t(12;13) associated with AML results in expression of high levels of CDX2 from the
TEL locus.
42 CDX2 is a homeotic protein that regulates expression of HOX family members in the colonic epithelium. As in
hematopoietic development,
HOX gene expression is highest in colonic stem cells in the colonic crypts and is down-regulated with maturation. It has been shown that CDX2 and CDX4 can dysregulate HOX expression in hematopoietic progenitors and result in leukemia.
42,
43 Evidence to support this includes the ability of CDX2 to induce leukemia in murine retroviral transduction models.
42,
43 Although CDX2 is not normally expressed in hematopoietic cells, a family member, CDX4, has been cloned and appears to play a similar role in hematopoietic development as CDX2 does in the gut. Of note, CDX4 in hematopoietic cells appears to either be downstream or epistatic with MLL in regulation of
HOX gene expression.
44Taken together, these data indicate that the NUP98/HOXA9 fusion transforms hematopoietic progenitors in part through dysregulated overexpression and by transactivation mediated through the NUP98 transactivation domain that recruits CBP. However, like other gene rearrangements involving hematopoietic transcription factors, expression of NUP98/HOXA9 alone is not sufficient to cause leukemia. In murine bone marrow transplant models, NUP98/HOXA9 induces AML only after markedly prolonged latencies indicative of a requirement for second mutation.
CHROMOSOMAL TRANSLOCATIONS THAT TARGET THE MLL GENE
As noted earlier in “Recurring Chromosomal Abnormalities in Acute Leukemia,” the
MLL locus is involved in more than 40 different chromosomal translocations with a remarkably diverse group of fusion partners,
45,
46 and are associated with mostly FAB subtype M4 or M5, and fewer with M2 AML. Patients who have received prior chemotherapy for cancer and develop AML (therapy-related myelodysplastic syndrome [MDS]/AML, t-AML) often have abnormalities in 11q23, especially those patients treated with topoisomerase inhibitors such as etoposide or topotecan. Chromosomal translocations involving band 11q23 result in expression of a fusion gene containing amino-terminal
MLL sequences fused to a wide variety of partners. There has been no common functional motif or activity ascribed to all partners; however, specific fusions may be associated with specific leukemic phenotypes. The MLL/AF4 fusion associated with t(4;11) is frequently observed in infant leukemias and is associated with an ALL phenotype in more than 90% of cases, whereas the MLL/AF9 fusion associated with the t(9;11) is almost exclusively associated with AML. Certain MLL fusion genes also have prognostic significance. For example, patients with t(9;11)(p22;q23) have a better outcome than those with other translocations involving 11q23.
The
MLL gene encodes a large, ubiquitously expressed protein. The
Drosophila protein trithorax, a homologue of
MLL, regulates patterning and
HOX gene expression during development. It has been hypothesized, in part based on these observations, that MLL might be required for maintenance of
HOX gene expression. In support of this hypothesis, mice that lack
Mll express HoxA7 but are not able to maintain its expression.
47 Mice that have homozygous deficiency for
Mll have an embryonic lethal phenotype at day 10.5 postconception. Even heterozygous animals have developmental anomalies in the axial skeleton and hematopoietic deficits including anemia.
47 Thus, as for other genes targeted by chromosomal translocations,
MLL is important for normal hematopoietic development.
The function of
MLL is not fully understood, but cell culture and murine models have provided some insight into transforming activity of the fusion proteins. The MLL protein of the fusion protein retains the amino terminal AT hooks that facilitate binding to DNA, as well as a methyltransferase domain. With the exception of CBP/p300, the function of the remaining broad spectrum of divergent fusion partners is poorly understood. In fact, the remarkable divergence of partners has suggested that alteration in the
MLL gene itself is a critical required event for transformation. In support of a central role of
MLL rearrangement in AML, it has been reported that partial tandem duplications of
MLL are associated with AML, in particular AML associated with +11.
48 Data demonstrating that MLL is a processed polypeptide provide further support for this hypothesis. MLL is processed by proteolytic cleavage into two component parts by a novel protease called
taspase 1.49 Cleavage is required for normal regulation of expression of anterior and posterior
HOX gene paralogs during development. It has thus been suggested that
MLL fusion genes, which are not cleavable, may mimic the uncleaved native MLL protein, thereby dysregulating
HOX gene expression.
50