Cushing’s syndrome signs and symptoms
Common symptoms
Physical exam signs
Less common symptoms
Physical exam signs
– Weight gain
– Dorso cervical (Buffalo) hump
– Supra clavicular fat
– Central weight with extremity sparing
– Skin changes
– Difficulty with wound healing
– Increased infections
– Easy bruising
– Skin lesions and ulcerations, thin skin, telangiectasias, purpura
– Ecchymosis
– Violaceous abdominal/breast striae
– Hyperpigmentation/acanthosis nigricans
– Skin tags (acrochordons)
– Kyphosis
– Height loss
Facial changes
– Acne
– Rounding
– Red facial flushing
– Facial plethora
– Acne
– Moon facies (rounding)
– Vision changes
– Blurred vision
Macroadenomas
– Visual-field defects (often bitemporal)
– Excess hair growth on face, neck, chest, abdomen and thighs
– Swelling of feet/legs
– Overall weakness and fatigue
– Hip and shoulder weakness
– Hirsutism/breast, back, abdominal hair
– Alopecia/frontal balding in woman
– Edema/generalized/LE
– Loss of muscle mass
– Proximal muscle weakness
Psychological problems
– Irritability
– Poor concentration and short-term memory
– Anxiety
– Mood swings
– Sleep disturbance and insomnia
Less common
– Glucose intolerance or diabetes mellitus
– Hypertension
– Hyperlipidemia
– Osteopenia/osteoporosis
– Menses irregular/absent
– Decreased fertility and/or libido or impotence
– Breast discomfort and discharge
– Decreased testicular volume in men
– Galactorrhea
In children
– Growth retardation
The prevalence of CD is estimated at 2.4 cases per million with an incidence of 1.2–2.4 cases per million per year. Women are more commonly affected than men.
Although a patient may be phenotypically “cushingoid,” the etiology of excess hypercortisolism varies. The most common cause, by far, is the use of exogenous steroids for the treatment of an underlying disease. When this and factious hypercortisolism are excluded, other sources of pseudo-Cushing’s need evaluation including anorexia, malnutrition, poorly controlled diabetes mellitus (DM), and glucocorticoid resistance. Once these are eliminated other endogenous etiologies should be evaluated and are generally divided into ACTH-dependent and ACTH-independent causes. ACTH-dependent cases account for approximately 80 % of all cases: 80 % of these are the result of a corticotroph producing pituitary adenoma and represent CD, while roughly 20 % are due to ectopic ACTH production. ACTH-independent CS is largely associated with adrenal adenomas and autonomous cortisol production.
Cortisol excess predisposes the individual to increased risk of cardiovascular disease and hypertension, impaired glucose tolerance and DM, impaired immune function, carotid intimal changes, clotting disorders, and osteoporosis with associated morbidity and mortality risks.
Screening and Confirmatory Tests for Hypercortisolemia
When CS is suspected, clinicians are frequently faced with a complex symptomatology and perplexing laboratory tests: any evaluation is compounded by the complicated physiology of cortisol and ACTH, which are increased in patients who are stressed. These patients without CS can have mildly elevated 24-h UFCs while conversely, patients with mild CS may have normal 24-h cortisol production rate.
A 1 mg overnight dexamethasone suppression test and a midnight salivary cortisol level are good screening tests. Normal cut-off values for the overnight suppression test have decreased over time from an a.m. cortisol level of <5 to <1.8 μg/dl, which increases sensitivity and decreases specificity. 24-h UFC represent also an important screening method, but could be normal in cases of early disease or recurrence.
In the case we present, additional studies were performed to rule-out elevated cortisol levels associated with pseudo-Cushing’s, caused by other underlying diseases such as active depression, alcoholism, obesity, and PCOS. A dexamethasone suppressed CRH test is most commonly used to distinguish CS from pseudo-Cushing’s and in our case, cortisol levels were >1.4 μg/dl at 15 min after the administration of intravenous CRH (the cut of indicative of CS). Some recent reports have recommended a cut-off for cortisol of >2.5 μg/dl (or >20 % over baseline) and an ACTH increase of >35–50 % at 15 min after CRH administration as a better discriminator. It is important to remember that no one test is 100 % sensitive and/or specific to determine if a patient has true CS or pseudo-Cushing’s; therefore, physicians must make every attempt to avoid a misdiagnosis. At our center, we follow a conservative approach and do not proceed to imaging (or surgery) unless we are confident of a biochemical diagnosis. This can justifiably cause frustration for patients who are often troubled by absence of an explanation for their symptoms. Conversely, as CS is a progressive disease, it is important to follow-up initial negative or borderline results in 6 or 12 months to make sure that no patient with CS is overlooked.
Localization of ACTH Excess
Brain magnetic resonance (MR) imaging in most, but not all, cases of CD reveals evidence of a pituitary adenoma; residual tumor was apparent in the case we present and the patient was referred to a neurosurgeon for further transsphenoidal resection of a pituitary microadenoma. Three months postoperatively, MR imaging did show postoperative changes, but no tumor was identified.
Pituitary adenoma in a patient with CS could represent an incidentaloma, thus bilateral inferior petrosal sinus sampling (IPSS) is considered the gold standard to differentiate between pituitary and ectopic sources of ACTH. A ratio of petrosal sinus to peripheral vein serum ACTH levels of >2–3:1 μg/dl measured after CRH is injected into a catheter positioned in both inferior petrosal sinuses is indicative of CD. The sensitivity of this test in CD is reportedly 94 % with a specificity of 100 %. However, both false positive and false negatives have been reported and are complicated by technical and venous anomalies. Tumor lateralization is also aided by IPSS, but with less accuracy.
Confirmation of a diagnosis after an initial failed TSS is paramount. While we considered petrosal sinus sampling to confirm CD as opposed to ectopic ACTH production, repeated assessment of her outside surgical pathology by our pathologist established the presence of a corticotroph adenoma at the first surgery.
Persistent Cushing’s Disease
The consequences of persistent hypercortisolism are severe and include immunosuppression, poor wound healing, diabetes, high blood pressure, cardiac insufficiency, severe osteoporosis, and increased mortality. Hence, early identification of patients at risk of treatment failure is exigent. Diagnosing persistent or recurrent CD is perhaps even more challenging than making the initial CD diagnosis. Several investigators have recommended serial measurement of serum cortisol in the days after pituitary surgery to identify patients with persistent CD.
All therapeutic options were discussed with this patient’s case: medication, bilateral adrenalectomy (BLA), or repeat transsphenoidal surgery (TSS). Transsphenoidal surgery in the hands of an experienced surgeon has demonstrated remission rates of between 65 and 93 % when used as first-line therapy. However, even with immediate re-exploration, after nonremission, rates drop to about 50 %. Remission rates are lower for larger, invasive tumors. On this basis, medical therapy with ketoconazole was recommended for our patient. However, despite acknowledging low chances of remission, she opted for a second surgery and she was reluctant to use medical therapy even as a preoperative treatment. She underwent repeat TSS; however, despite resection by a very experienced surgeon, no adenoma tissue was identified.
Management/Treatment of Cushing Syndrome After Failed Surgery
The goals for treatment of patients with CS are; reversal of clinical features, normalization of cortisol levels, and long-term control without recurrence. The primary modality for definitive treatment is TSS. Recurrence rates vary widely and may be as high as 26 % during long-term follow-up. If surgery is unsuccessful or hypercortisolism recurs, second-line treatment options include repeat surgery, medical therapy, radiation, BLA, or a combination of these options (Fig. 3.1). Bilateral adrenalectomy has been described as a definitive cure for CD when pituitary adenomectomy fails to achieve remission. However, it falls far short of an ideal treatment. Tumor corticotroph progression has been reported in 39–47 % of patients within a median follow-up of 4.6 years but predictive factors have remained elusive. High ACTH levels associated with tumor progression can be accompanied by the development of marked skin hyperpigmentation tumor enlargement and visual field deficits. In one CD study, 50 % of patients continued to report persistent adverse symptoms despite BLA. Early identification of corticotroph tumor progression is possible with close monitoring with MR imaging and ACTH levels and further surgical resection and/or radiation therapy is usually indicated.
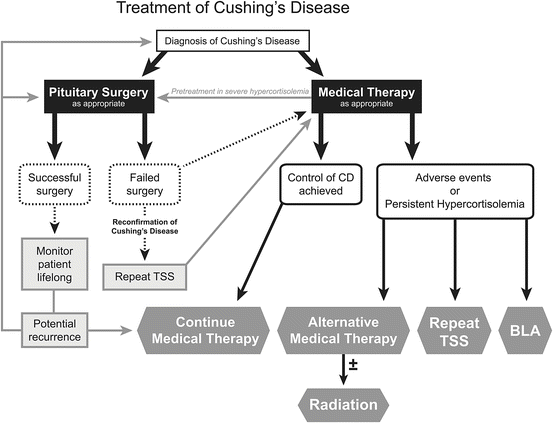

Fig. 3.1




Cushing’s disease treatment options. Reprinted from Neurosurg Clin N Am volume 23(4);653-668, Fleseriu, M., Medical management of persistent and recurrent cushing disease, page 655, Copyright (2012), with permission from Elsevier
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree