where S is a permeability coefficient.
Therefore, increased net filtration can result from any of the following or a combination of (1) increased capillary permeability, (2) increased surface area, (3) increased hydraulic pressure differences, and (4) decreased oncotic pressure differences.
Increased Capillary Permeability
Murine models of carcinomatosis have revealed increased permeability of proteins compared to control animals, with peritoneal capillary permeability reaching three times normal within a week of intraperitoneal tumor instillation.13 This permeability is thought to be driven, in part, by tumor angiogenesis, as in the process of neovascularization the basement membrane and stroma are remodeled and there is a period of hyperpermeability during the migration and proliferation of new endothelial cells.14 Multiple growth factors are critical to the process of angiogenesis, including fibroblast and vascular endothelial growth factor (VEGF), and early murine models of MA found increased VEGF (termed vascular permeability factor at the time) in the ascitic fluid proportional to the amount of neovascularization.15 The MA could be inhibited by treating mice with anti-VEGF antibodies, which reaccumulated upon withdrawing the antibodies.16 These findings have been reflected in patients with MA where marked neovascularization of the parietal peritoneum was observed,17 and ascitic fluids were found to contain high levels of VEGF, especially in patients with gastric, colorectal, and ovarian cancers compared to ascites from patients with nonmalignant ascites due to cirrhosis.18–20 The effect of VEGF on vascular permeability is estimated to be 50,000 times more potent than histamine.20
Another factor that may increase vascular permeability in these patients is the increased production and secretion of matrix metalloproteinases (MMP) by tumor cells. These enzymes, namely gelatinase and stromelysin-3, function to digest extracellular tissue scaffolds to allow tumor in-growth.21–23 Inhibition of these enzymes can reduce MA formation, and in a murine model of colon and ovarian MA, not only was ascites formation inhibited, but survival was increased.24,25 Therefore, carcinomatosis appears to increase capillary permeability through a process of growth factor–driven angiogenesis and MMP production, and this may play a role in increasing intraperitoneal fluid and macromolecules.
Increased Surface Area
As the surface area of capillaries increases, so does the potential amount of cell permeate. Secondary to angiogenesis and growth factor–stimulated neovascularization, the size and amount of mesothelial blood vessels is increased in a murine model of MA.26 Furthermore, in patients with carcinomatosis, studies measuring the sources of ascitic effluent by using plastic rings and absorptive paper on both tumor surfaces and tumor-free surfaces, found an abundance of effluent from nontumor-bearing omentum and small bowel, thus identifying additional surfaces not traditionally considered responsible for ascites formation.27,28
Increased Hydraulic Pressure Differences
Increased portal pressures can increase the hydraulic pressure in the mesothelial capillaries. Though this does not seem to play a large role in the formation of MA, increases in portal venous pressure have been seen in patients with ovarian cancer with MA.28 Furthermore, malignancies that cause liver dysfunction due to excessive hepatic tumor load or form on a background of cirrhosis (e.g., hepatocellular carcinoma) can tip the balance of hydrostatic pressures in the mesothelial capillaries to levels above the interstitium.
Decreased Oncotic Pressure Differences
Chronic or advanced malignancy can result in chronic protein malnutrition. This results in a catabolic state with reduced intravascular protein, thereby creating an oncotic pressure gradient that favors fluid extravasation from the vessels into the peritoneal cavity and lack of fluid reabsorption.29 In addition, due to the increased capillary permeability in patients with carcinomatosis, proteins and other macromolecules enter the ascitic fluid and accumulate in the intraperitoneal cavity.20,30 A combination of these forces increases the extravascular oncotic pressure, driving fluid into the interstitium.
Increased Ascitic Fluid Production
Increased fluid production appears to contribute to the formation of MA secondary to increased microvascular permeability, increased surface area, increased hydraulic pressure, and decreased colloid oncotic pressure. Capillary permeability is increased due to cytokine release. Molecules such as VEGF, vascular permeability factor, interleukin-6, and tumor necrosis factor (TNF) are intimately involved.4 Enhanced vascular permeability leads to secretion of osmotic particles and fluid into the peritoneum, further increasing the intra-abdominal fluid volume. Endocrine homeostasis may also contribute. Intravascular volume is decreased with increasing ascites, which results in pressure-volume feedback loops that stimulate renin and aldosterone secretion. These hormones reduce urinary output and incidentally increase accumulation of ascites.4,31 Furthermore, due to decreased removal of fluid as a consequence of malignant obstruction of lymphatics, the circulating blood volume is further reduced and this similarly activates the renin–angiotensin–aldosterone system.
Reduction in Fluid Reabsorption
Venous and lymphatic obstruction by visible solid tumors or by microscopic tumor cells is one mechanism that results in impaired drainage of peritoneal fluid content.32 Fluid kinetic studies performed on patients undergoing peritoneal dialysis identified that the normal rate of lymphatic uptake remains constant at 40 ml/h.33 This rate would be necessary in the absence of increased production to maintain homeostasis. Murine models of carcinomatosis by intraperitoneal tumor cell injection resulted in diaphragmatic and retrosternal lymph occlusion by 5 days with ascites forming soon thereafter. Lymphoscintigraphy in human patients with MA also documented no radioactive sulfur colloid above the diaphragm in 85% of patients treated with tagged intraperitoneal colloid, implying that diaphragmatic lymphatics were obstructed by malignant invasion.13,34–36 Additionally, due to increased capillary permeability, proteins and other macromolecules enter the ascitic fluid and these can mechanically obstruct lymphatic drainage channels.20 Therefore, the rate of lymphatic uptake may be decreased or inhibited in malignancy, contributing to increased intra-abdominal fluid.
Comprehensive history taking combined with physical exam will diagnose ascites in most patients. In patients with known malignancy, increasing abdominal girth, bloating, abdominal discomfort, or weight gain are concerning for carcinomatosis and MA. Imaging, usually computed tomography (CT) scan or ultrasound,37 can confirm the presence of ascites (Fig. 128.1). The CT finding of peritoneal nodules, thickening, omental caking, solid organ metastases, or intraperitoneal nodules raise the suspicion that ascites is malignant as opposed to secondary to benign etiologies. In over 100 patients studied with MA or cirrhotic ascites, CT signs significantly associated with a malignant etiology were fluid in the omental bursa, higher ascitic density (11.5 HU versus 6.7 HU), thickening of the parietal peritoneum, and, in patients with at least a moderate amount of ascites, a tethered-bowel sign.38 As most patients with abdominal pain or malignancy are undergoing cross-sectional imaging as part of the workup or for surveillance, attention to these radiographic signs are important as they can help direct treatment and streamline further workup.
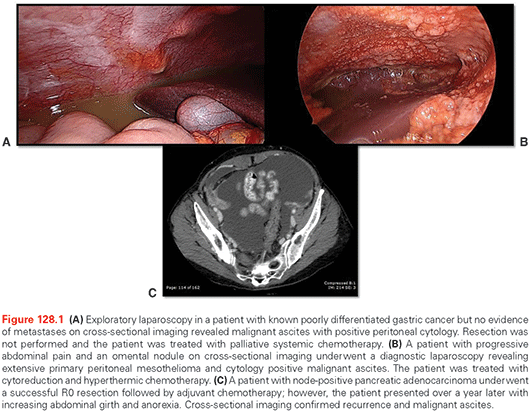
The gold standard to diagnose MA is positive peritoneal cytology, which is present nearly 100% of the time in patients with carcinomatosis.39 This can be obtained percutaneously and safely under image guidance. For advanced gastrointestinal and hepatopancreaticobiliary malignancies without metastases on cross-sectional imaging, diagnostic laparoscopy is often utilized for evaluation of occult carcinomatosis (see Fig. 128.1). If incidental low-volume ascites is found in these patients, then ascites can be aspirated and set for cytology, as can peritoneal washings. This is now a standard approach for advanced gastric cancer in some centers, including our own, as positive peritoneal cytology carries a prognosis similar to stage IV disease.
For those without carcinomatosis but still with MA, cytology is diagnostic in only 50% to 60% of patients.40 For these patients, perhaps the most useful single test is the serum to ascites albumin gradient. Calculated as the difference between serum and ascites albumin concentrations, values <1.1 g/dl are suspicious for carcinomatosis as opposed to portal hypertension or other cardiopulmonary causes.41 The use of ascitic fluid protein and other chemistries to discriminate between malignant and nonmalignant causes is variable and not reliable. If negative for malignancy, cell counts with >250 neutrophils per mm3 are suggestive of infection and Gram stain should be performed. High levels of ascitic amylase may reflect pancreatic ascites or small bowel perforation.42 Ascitic fluid fibronectin levels have been reported to be 100% sensitive and specific for malignancy, but this is not a standard test currently, nor is sialic acid, telomerase, or human gonadotropin-beta, all of which have shown discriminatory promise.43–46
In the absence of a known primary tumor or imaging revealing evidence of malignancy, serum tumor markers can be helpful. Elevated carcinoembryonic antigen values are traditionally associated with colorectal cancer, but can also be elevated in breast, lung, pancreas, gastric, or breast cancers. Hepatopancreaticobiliary tumors can often reveal elevated Ca19-9 levels. Increased Ca125 levels are most suspicious for ovarian carcinoma, though they can be elevated in pancreas, lung, and breast cancers. Alpha-fetoprotein is another helpful serum marker, which when elevated can be indicative of hepatocellular carcinoma.
The management of MA has evolved from the contributions of many specialties, including surgical oncology, medical oncology, hepatology, palliative care, and interventional radiology. The goals of treatment are to provide symptomatic relief and to either maintain or improve the quality of life. Randomized controlled trials are lacking, and studies evaluating the efficacy of various treatments are difficult to extrapolate to the individual patient. Common end points have included comparisons in time between paracenteses. Therefore, there is no standard approach to these patients and multiple medical and surgical options exist. The mainstays of treatment have been based on non-malignant acsites and include diuretics and paracentesis.4 A survey of randomly sampled Canadian medical oncologists, gastroenterologists, palliative care physicians, and gynecologic oncologists revealed that 98% employed paracentesis with 89% efficacy, followed by diuretic use 61% of the time and rarely peritoneal shunting—both with unclear advantages.47 Certainly, systemic antineoplastic therapy should be utilized whenever possible in a malignancy-specific fashion. In the last few years, new systemic agents as well as regional chemotherapy combined with surgical cytoreduction have offered alternative options for palliation. The various options for treatment are reviewed herein.
Removal of Fluid
Perhaps the most utilized treatment strategy in managing MA is by removal of fluid, either by diuresis, paracentesis, or shunting.
Diuretics
The use of diuretics in the management of MA is largely extrapolated from the treatment of cardiogenic or cirrhotic ascites, though it has been advocated as first-line treatment of MA.48 Its role appears to be most useful when the etiology of ascites involves stimulation of the renin-aldosterone axis.8,49 As a result, diuretic use is most successful in selected patients with liver dysfunction or portal hypertension secondary to metastatic liver disease or extensive intrahepatic primary tumor burden, as opposed to patients with peritoneal disease or superficial disease of Glisson capsule.40,49 For this reason, patients with a serum to ascites albumin gradient <1.1 (see the section titled “Diagnosis”) have been found to be less responsive to diuretic use, as was identified in a prospective study of patients with MA of various primary tumor etiologies.49 A review of five studies with >100 patients with MA from a variety of primary tumors found the use of diuretics to be successful in managing symptoms in up to 43% of patients.50 The agent most commonly utilized is spironolactone with doses ranging from 150 to 450 mg/day.51 Loop diuretics (e.g., furosemide) can be added to spironolactone.4 Maximal expected ascitic reabsorption can reach 800 ml with optimized medication doses in selected patients, though it was noted by Pockros et al.49 that no change in ascitic volume was achieved in patients with MA secondary to peritoneal carcinomatosis or chylous malignant ascites. Complications of diuretic use include dehydration, renal toxicity, and electrolyte imbalances that can result in cardiac arrhythmias. There is no level 1 data evaluating diuretic efficacy in MA and, as a result, its use is controversial and without clear guidelines.47
Paracentesis
Paracentesis is the most commonly used treatment for patients with MA with up to 98% of physicians utilizing it for their patients.47 Improvement in symptoms, although temporary, can be achieved in ~90% of these patients,24 and its use in palliation is supported by the available literature.52 There are no randomized trials comparing paracentesis with the use of diuretics in the management of MA.
The volume of drainage per session is limited by the potential complications of hypovolemia and resulting hypotension or renal dysfunction, though hypovolemia and hypotension may be averted with concomitant 5% dextrose infusions or intravenous albumin.53–57 Randomized studies have demonstrated no difference in survival between patients treated with albumin compared to other plasma expanders, and for patients with MA no definitive trials on this subject have been performed.58 Reports of 5 L per 1 to 2 hours were demonstrated to be safe without hypovolemia or renal dysfunction,50 and volumes up to 20 L per session have been reported, though there is no consensus on the rate of withdrawal, ranging from 30 minutes to 24 hours.54,56,59–61 Forty-eight paracenteses in 44 patients with MA suggested that removal of a mean of 5.8 L (range, 0.8 L to 15 L) can provide palliation of symptoms.55 Serial paracentesis can result in hypoalbuminemia, a complication possibly minimized with high-protein diets and small volume taps.62,63 Additional procedural risks of paracentesis include solid and hollow viscous organ injury or perforation, bleeding, fistula, peritonitis, and sepsis. In patients with carcinomatosis and/or prior surgery, there may be extensive scarring and fluid loculations, making ultrasound-guided paracentesis attractive with potentially less complications in this population. As the procedure is often required on multiple occasions,47 efficacy decreases and complication risks compound. In a study of 67 patients with ovarian, breast, and colorectal cancer, 392 paracentesis, or approximately six aspirations per patient, were required for palliation with other studies reporting mean procedure-free intervals to be 10 to 11 days.50,64
To overcome the risks, potential complications, and patient discomfort of serial paracentesis, indwelling peritoneal drainage catheters may be advocated. Furthermore, these catheters offer the option of self-drainage. The utility of catheter placement should be weighed against the patient’s condition as survival from catheter placement ranges from 15 days to 18 months.65 Typically, patients with a life expectancy of >2 to 3 months and requiring serial taps are considered for this procedure.66 The most frequently used options include tunneled Tenckhoff catheters (Covidien, Mansfield, MA), untunneled or tunneled PleurX catheters (Denver Biomedicals Inc., Golden, CO), cope-type loop catheters, and tunneled peritoneal Port-a-Caths (Smiths Medical, St. Paul, MN).65 The greatest risk with indwelling catheters is peritonitis, which occurred in 4.4% of patients with tunneled Tenckhoffs67 and 2.5% of tunneled PleurX catheters compared to reports of 21% to 34% for untunneled catheters.68
Additional complications can occur in ~25% of patients with untunneled catheters including leakage from the insertion site, cellulitis, infections, occlusion, and fatal hypotension.4,47,64,65,67,69 Tunneled catheters appear to have a superior efficacy and patency rate of >95%.69 Control of ascites ranges from 83% to 100% of cases with a mean patency duration of 52 days.4 It should be noted that though these catheters are widely used to treat MA with successful outcomes, it remains off-label use.
Shunting
Shunting involves removal of fluid from the peritoneal cavity and returning it to the venous circulation (superior vena cava) with the goal of maintaining electrolytes and proteins, as compared to drainage procedures. The most commonly employed options include the LeVeen catheter and the Denver shunt (Denver Biomaterials Inc., Denver, CO)70 (Fig. 128.2). Peritoneovesicular shunts were attempted; however, peritonitis and occlusion limited their utility.71 There is no level 1 evidence comparing shunt efficacy in MA.50 Contraindications to shunt placement include hemorrhagic ascites, loculations, heart disease, renal failure, jaundice, portal hypertension, pleural effusion, and clotting disorders.4,66 Theoretically, there is a risk of systemic seeding from positive peritoneal cytology, but this has not been found to be clinically signficant.66,72
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree