• A review of 165 studies found that stress-related psychosocial factors are associated with higher cancer incidence in initially healthy populations, poorer survival in patients diagnosed with cancer (330 studies), and higher cancer mortality (53 studies) [19]
• Stress-prone personality, unfavourable coping styles, and negative emotional response were related to higher cancer incidence, poorer cancer survival and higher cancer mortality [19]
• The Finnish Twin Cohort study found an association between the accumulation of life events during 5 years prior to baseline assessment and increased risk of breast cancer: divorce, separation, death of a husband/close relative/friend were all associated with increased risk of breast cancer [63]
• Prior stressful experiences in childhood have been linked to decreased cellular immunity and depressive symptoms in breast cancer survivors [28]
There is also a link between psychological distress such as anxiety and depression, and cancer. Depression is common in cancer patients, affecting up to 25% [46, 64]. There is evidence to indicate that depression may be associated with an increased risk of cancer and may predict cancer progression [92, 108]: the longer the duration of depression, the greater the risk of developing cancer [85]. Risk of developing cancer due to depression is almost doubled, independent of other lifestyle factors, and this risk is not related to any specific cancer [92]. Depressive symptomatology has been found to be a consistent psychological predictor of decreased survival time [17].
Coexistence of Anxiety and Depression in Cancer
Anxiety and depression often coexist in cancer patients. In a large epidemiological study of women diagnosed with breast cancer, 10.8% had combined anxiety/depression symptoms (CADS), 14.9% had only anxiety symptoms and 2.8% had only depressive symptoms [16]. Another study found that 44.5% of women with breast cancer were diagnosed with CADS, and that higher levels of anxiety with or without sub-syndromal depressive symptoms were associated with higher fears of recurrence and decreased life satisfaction [40]. In addition, both anxiety and depression have also been found to be associated with cancer-related fatigue, a symptom that affects a significant percentage of cancer sufferers [47]. And as Kotsirilos and colleagues point out [56], depression can also influence lifestyle factors that may negatively impact on health including smoking, consuming an excess of alcohol, poor level of physical activity and excess weight [45].
Table 2.2 sets out some of the facts and figures about the relationship between anxiety, depression and cancer.
Table 2.2
Some facts and figures about anxiety, depression and cancer
• Depression prevalence in cancer patients has been found to increase with disease severity and symptoms such as pain and fatigue [108] |
• There is some evidence that depression predicts cancer progression and mortality (though this is complicated by several factors including that some cancer symptoms and cancer treatment can mimic depression, and disease progression can have a negative effect on mood) [108] |
• Perceived stress, anxiety and pain severity have been found to be significantly associated with greater severity of cancer-related fatigue [47] |
Key Components of Cancer Pathogenesis
Before we take a look at how the mechanisms of how stress may create changes at the cellular level that may contribute to cancer, we will look briefly at the key components of the microenvironment associated with cancer, or the ‘hallmarks of cancer’. These are the (so far) known pathogenic events that are present in cancer. These include: sustaining proliferative signalling, evasion of growth suppressing signals, resistance to apoptosis (programmed cell death), replicative immortality, ability to induce angiogenesis, ability to invade and metastasise, ability to evade destruction by the immune system and a reprogramming of energy metabolism, underpinned by genome instability and inflammation [44].
Inflammation is a key aspect of the biochemical environment associated with tumors, as it is with other chronic diseases including diabetes and other cardiovascular diseases. Lung, colorectal and breast cancer have all been found to be associated with high levels of C-reactive protein, an inflammatory marker and a stronger association has been found between increased levels of inflammatory markers and risk of cancer death compared with the risk of cancer incidence [49].
Sex hormones have also been implicated in the pathogenesis of some cancers such as breast and prostate cancer, though for prostate cancer, there is mixed evidence for the role of testosterone in carcinogenesis [75].
Insulin and IGF, and oxidative stress are also involved. In cancer, oxidative stress and the production of free radicals occur at a much higher rate in cancer cells than normal cells [9]. Increased insulin can stimulate tumor development and progression, including cancer cell proliferation and migration in cancer cell lines [26] and has been found to have mitogenic and anti-apoptotic effects in endometrial cancer [119]. Oxidative stress can increase mutagenesis and DNA mutation rate and upregulate angiogenesis, disrupt mitochondrial functioning (causing fatigue) and accelerate tumor sculpting [9].
What is happening at the cellular level is obviously very complex. The reader is referred to other sources for detail on the cancer pathogenesis.
The Cellular Level: Stress, Endocrine and Immune Systems and Cancer
Stress and depression influence the body through the brain: psychoneuroimmunology (PNI) is the study of how the mind influences the immune system (to be normal, abnormal or hyperactive), and psychoneuroendocrinology (PNE) describes how the mind influences the body’s endocrine system [103]. Inflammation, a key process in cancer and other chronic diseases, is modulated via bidirectional communication between the neuroendocrine system, immune system and the brain [118], with increasing evidence of the role of the gut microbiome (more later).
Stress is able to activate the autonomic nervous system and the hypothalamic-pituitary-adrenal (HPA) axis and thereby alter the tumor microenvironment. When stress activates the HPA axis, corticotrophin-releasing hormone (main regulator of HPA axis) is secreted and this induces the release of adrenocorticotrophic hormone into the systemic circulation, where it stimulates the adrenal cortex to produce glucocorticoids. Glucocorticoids (e.g. cortisol) along with catecholamines (noradrenaline and adrenaline) from activation of the autonomic nervous system are released into the bloodstream as part of the stress response, as are inflammatory cytokines [23, 68]. These can all affect the tumor microenvironment. Glucocorticoids can also directly mediate processes that promote tumor growth, activate survival genes in cancer cells, down-regulate repair genes and inhibit the cellular immune response [68]. Stress has also been shown to influence many parts of the steps involved in metastatic spread of cancer [68].
Stress and the Immune System
Chronic stress, via activation of the HPA axis, results in the release of various mediators that can suppress some of the non-specific and specific parts of the immune response including NK-cell activity, phagocytosis, production of inflammatory cytokines (e.g. IL-2, interferon, TNF by Th1 cells) and activity of cytotoxic T cells, all of which are important components of the cancer surveillance and the immune response against cancer [68, 99]. Psychological stress also reduces the ability of cells to initiate genetically programmed cancer cell death [116].
Chemical messengers secreted by nerve cells, endocrine organs or immune cells mediate the communication between the central nervous system (CNS) and the immune system, and psychological stress can down-regulate various parts of the cellular immune response, disrupting these communication networks between the CNS and immune system [99]. Bio-behavioural oncology research has found that stress can not only down-regulate the cellular immune response (mediated largely by adrenergic and glucocorticoid signalling) but that it can also affect tumor angiogenesis, invasion and anoikis, stromal cells in the tumor microenvironment and inflammation, all pathways involved in cancer [68].
Interventions that reduce psychological distress have been found to create changes at the cellular level, for example, significantly increasing the percentage of large granular lymphocytes and increasing NK cytotoxic activity in patients with malignant melanoma [31].
Stress and Chronic Inflammation
There is strong evidence that chronic inflammation precedes tumorigenesis [59] and that the stress response and inflammation play an important role in the pathogenesis of cancer metastasis [68, 91]. Chronic stress can lead to inflammation through the production of cytokines, including interleukins [68, 99]. People with major depression have been found to have increased production of pro-inflammatory cytokines interleukin 1, interleukin 6, soluble interleukin 2 and interleukin 6 receptors, suggesting that concentrations of pro-inflammatory cytokines correlate with HPA activity and disease severity [99]. Stress increases the level of cytokines such as Vascular Endothelial Growth Factor (VEGF), a cytokine which mediates angiogenesis and cancer patients with high levels of VEGF have a poorer prognosis [45]. Cytokines can also stimulate tumor growth [45].
Stress and the Endocrine System
Stress and depression can alter the functioning of the endocrine system, leading to increased insulin secretion from the pancreas, increased cortisol, growth hormone and prolactin, which can contribute to increased tumor growth [56]. Neuroendocrine stress hormones, released via the brain, sympathetic nervous system and/or HPA axis, in the tumor microenvironment assert a systemic influence on tumor growth [68]. High cortisol levels (and a tendency towards flatter diurnal cortisol rhythms) have been found to be associated with greater disease severity in women with metastatic breast cancer [1]. Other research in women with early metastatic breast cancer found that women who repressed emotions and those who were highly anxious had a significantly flatter diurnal cortisol slope than self-assured and non-extreme groups, though there was no difference in mean cortisol levels [36].
In patients with depression, cortisol was found to be increased in those who were chronically stressed, suggesting that depression is associated with sensitisation of the HPA axis to chronic stress [110]. As previously stated, depression affects a significant proportion of cancer patients [22, 46 and 64].
Corticotropin-releasing factor (CRF) is a hypothalamic neuropeptide that is involved in the control of the stress response. It is also expressed in organs and peripheral tissues [6]. In the tumor microenvironment, it may be produced by innervating sympathetic neurons, immune cells and endothelial cells [5, 6]. CRF has been found in breast cancer cells and tissue and can affect breast cancer cells in an autocrine or paracrine manner [5]. Research has found that CRF stimulates cell motility and invasiveness of breast cancer cells, and that the likely mechanism is via induction of Focal Adhesion Kinase (FAK) phosphorylation and the reorganisation of actin filaments and the production of prostaglandins: CRF induces the production of prostaglandins and expression of Cox-1 in breast cancer cells, which are factors that can promote invasiveness and metastasis [5]. In vitro, research has found that CRF can induce the expression of genes involved in breast cancer proliferation and metastases. In vivo, research has also demonstrated that peripheral CRF at least partly mediates the tumor-promoting effects of stress (including neoangiogenesis and tumor growth) [6].
Stress and Genetic Damage
Stress can lead to DNA damage, accumulation of somatic mutations, impaired genetic mutation repair and inhibition of apoptosis [39, 50, 99]. In human studies, perceived psychological stress and perception of inability to alleviate stress have both been found to be associated with increased levels of 8-hydroxydeoxyguanosine (8-OH-dG), a biomarker of cancer-related oxidative DNA damage, in females but interestingly not in males [50]. In healthy workers, the levels of 8-OH-dG were higher in females with poor stress-coping behaviours and in males with a self-blame coping strategy [51].
At the Cellular Level: Stress, Depression, Anxiety and the Gut
The gut microbiome is assuming a much greater importance than previously given credit for in the stress response. The interaction between stress, the HPA axis and immune system is well established, and it is now believed that the gut microbiota mediates this response [23].
The newborn child is exposed to the maternal vaginal microbiota during childbirth, providing the main source for normal gut colonisation, host immune maturation and metabolism [52]. Microorganisms within the gut form part of a complex and multidirectional communication network with the brain, the microbiome-gut-brain axis [106]. The gut contains as many neurons as the spinal cord and there are many hormonal connections [42]. The microbiota and host, the human, have co-evolved into a complex ‘super-organism’ which has been beneficial to the host, but it can also create problems when the microbiota becomes dysregulated or disturbed. Each organ has a microbiome; however, 99% of the microbial mass is in the gastrointestinal tract [105].
The Gut and the Immune System
The gut microbiome is now understood to play a critical role in the functioning of the immune system, thereby influencing inflammation, as well as the nervous system [93]. The gut microbiome plays a key role in regulating intestinal permeability and maintaining the intestinal barrier function and deficits in intestinal permeability may be a causal factor in the chronic low-grade inflammation observed in depression [54]. A U.S. study found that major depression is accompanied by activation of the inflammatory response system and that pro-inflammatory cytokines and lipopolysaccharide (LPS) may induce depressive symptoms—a significant increase in level of antibodies against LPS in the blood was found in those with major depression [71]. LPS, the major component of the outer membrane of certain bacteria, is able to enter the bloodstream only if the normally tight junctions in the cells lining the intestine are compromised and the intestine lining becomes permeable or ‘leaky’ [93]. It can then fuel inflammation. Inflammation is a key component of many chronic diseases including cancer.
Regulation of Moods by the Microbiome-Gut-Brain Axis
The gut microbiome has been found to affect neural, immunological and endocrine systems, with the microbiome-gut-brain axis modulating emotions and moods. Stress is able to modulate the microbiota and the microbiota is able to alter the set point for stress sensitivity [106]. The gut microbiota generates several neurotransmitters including gamma-aminobutyric acid (GABA), acetylcholine, dopamine, norepinephrine and serotonin [42]. Neurotransmitter imbalances or deficiencies are known to cause psychiatric problems such as depression [42]; altered GABA receptor expression is implicated in the pathogenesis of anxiety and depression (both of which are associated with functional bowel problems) [15]. Depression has been found to be associated with dysregulation of the gut microbiota composition [106].
Animal research demonstrates clear links between the gut microbiome and stress/anxiety/depression as well as changes in the brain. This includes findings that the gut microbiota can modulate the blood-brain barrier [54]. How translatable such findings are to humans in understanding brain-gut relationships and disorders is not yet known [72, 73]. Some key findings from pre-clinical (animal) research are set out in Table 2.3.
Table 2.3
Pre-clinical (animal research): gut microbiome, stress, anxiety and depression
• Germ-free mice have an exaggerated HPA axis response to restraint stress which was reversed by moncolonisation with Bifidobacterium infantis [113] |
• Female mice stressed during pregnancy pass on lowered levels of gut bacterium to their pups [52] |
• Increased stress was found to be associated with decreased microbial diversity in the gut of wild red squirrels [111] |
• Absence of the gut microbiota in rats exacerbated behavioural responses to acute stress and was associated with an altered neurotransmitter turnover rate in areas of the brain known to regulate reactivity to stress and anxiety-like behaviour [20] |
• Short-term colonization of germ-free adult mice was found to reduce anxiety-like behaviours [84] |
• Treatment of mice with Lactobacillus rhamnosus (JB-1)-induced changes in GABA mRNA expression in areas of the brain and reduced stress-related cortiocosterone and anxiety and depression-related behaviour, with the Vagus Nerve implicated as the major modulatory pathway between the gut and the brain [15] |
• Germ-free adult mice exposed to faecal microbiota from pathogen-free donors had decreased blood-brain barrier permeability and increased expression of tight junction proteins [14] |
The relationship between the gut microbiome and emotions and stress is an important and complex one. However, there is increasing evidence that the gut microbiome and brain, immune and endocrine systems are all in constant communication, and the gut microbiome is a key player in the stress response. And the storage of emotions and stress is closely linked to the pathogenesis of cancer.
The Gut Microbiome and Cancer
There is increasing evidence that the bacterial microbiota plays a key role in carcinogenesis [105]. Whilst particular cancers are triggered by specific bacteria (e.g. gastric cancer and Helicobacter pylori), other cancers such as colorectal cancer appear to be triggered by tumor-promoting effects of microbiota (e.g. liver cancer may be promoted by pro-inflammatory micro-organism associated metabolic patterns (MAMPs) and bacterial metabolites from the intestine, via the portal vein) [105]. Colorectal cancer (CRC) has been found to be associated with decreased overall microbial community diversity and relative depletion of the Clostridia class of Firmicutes bacteria. Increased risk of CRC was found with increased carriage of Atopobium, Fusobacterium and Porphyromonas bacteria [3].
Use of antibiotics, known to disturb the gut microbiome, has been shown to be linked to an increased risk of breast cancer and of fatal breast cancer: a U.S. study demonstrated that there was a significant correlation between antibiotic use and terminal breast cancer [117].
Disturbances in Anatomical Barriers
Disturbances of the anatomical barriers between the host and microbes can also promote inflammation and diseases including cancer. The relationship is bidirectional: barrier failure can trigger inflammation, and inflammation and carcinogenesis can promote barrier failure [105]. These are but a few of the potential pathways of involvement of the microbiota; the mechanisms involved in carcinogenesis are complex. The reader is referred to other sources for a more complete explanation.
Obesity and Microbiotal Dysbiosis
Obesity, a major modifiable risk factor for the development of many types of cancer, is associated with microbiotal dysbiosis which may result in several physiological changes that could contribute to the link between obesity and cancer. These include altered microbial metabolism that can contribute to the generation of pro-carcinogenic toxic metabolites, induction of subclinical inflammation that could initiate tumor formation, and/or increased extraction of energy and nutrient availability causing metabolic dysregulation, thereby contributing to tumor growth [101].
The Gut Microbiome During Chemotherapy
The gut microbiome, of course, undergoes quite a beating during chemotherapy, with a severe compositional and functional imbalance in the gut microbiome associated with chemotherapy-induced mucositis [77]. Research has established that over the course of chemotherapy, there is a significant decrease in oral and intestinal microbial diversity and an increase in specific microorganisms known to cause infection [4]. A patient’s microbial diversity, even prior to cancer treatment, can be linked to increased risk of infection during induction chemotherapy. Thus researchers have suggested that microbiome sampling could be used to predict the chance of infections during chemotherapy, and that monitoring a patient’s microbiome during induction chemotherapy could be used to predict risk of microbial-related illness during subsequent treatments [4]. A depleted gut microbiome following chemotherapy leaves the patient with a greater risk of future illness.
Suffice to say, the gut microbiome is an important player in the pathogenesis of cancer. Given its role in influencing the immune, nervous and endocrine systems, including its role in depression which often coexists in cancer sufferers, addressing any imbalance in the gut will be important.
Social Support and Cancer
There is evidence of the impact of lack of social support in those with cancer. Tumors from high risk ovarian cancer patients (high levels of depression and low levels of social support) were compared with tumors from low risk patients (low levels of depression, high levels of social support)—it was found that tumors from high risk individuals showed more than 200 upregulated gene transcripts, many of which were involved in tumor progression. In addition, high risk patients had increased intra-tumor norepinephrine [67]. The association between storage of emotions and cancer is demonstrated in the findings of a systematic review that found that depression and constraint of emotions were associated with decreased survival in breast cancer patients [29].
Loneliness has been found to be related to higher levels of tumor vascular endothelial growth factor (VEGF) in patients with colon cancer [82]. In breast cancer patients, women with low levels of emotional expression and perceived emotional support were found to have poorer survival than women reporting high levels of both, and use of emotion-focused coping strategies was significantly associated with better survival [100]. A study of 9247 women with breast cancer found that women who were socially isolated women (small networks) were 1.43 times more likely to have a breast cancer recurrence, 1.64 times more likely to die from breast cancer and 1.69 more likely to die of any cause than socially integrated women [57]. Another study of people with cancer found that being unmarried carried a 27% and 19% higher risk of death in men and women, respectively, compared with their married counterparts [41].
Good News
The good news is that research indicates that social support can positively influence cancer survival, discussed in the later sections of this chapter.
What to Do About Stress in the Ultimate Consultation
In the Ultimate Consultation, the clinician’s role is to help the patient with cancer understand the link between the mind, stress and those processes in the body that contribute to poor health, and to harness the power of their mind to positively impact their health. The clinician can then guide the patient to several techniques that can help alleviate stress and in particular, unload the storage of stress and emotions.
The clinician will need to allow plenty of time for the consultation, in the order of 1–2 h. The benefit of taking this time will be tremendous, for both patient and clinician. Patients invariably are very appreciative of the opportunity to talk, in detail, with their clinician, and in many ways, this is part of the unloading of stored stresses and emotions itself. Importantly, this is an opportunity for both patient and clinician to understand what factors may have led to the patient becoming out of balance, and for the clinician to empower the patient to be proactive in not only changing those factors that can be changed, but also adopting other strategies to improve their well-being. Remember, the Ultimate Result (whatever that is for the patient, but ideally at the very least an improvement in health and well-being) really depends on the Ultimate Patient, one who is empowered and pro-active. The Ultimate Consultation is fundamentally about enabling and facilitating that empowerment.
Key Point
Allow plenty of time for the consultation, in the order of 1–2 h.
Beginning the Conversation About Stress
Mind Your Language
The language the clinician uses is most important. At the very outset, it is important to remember this is a person with cancer you are talking to, and the cancer is part of them, it originated due to imbalance within them, and it is a reflection of what is happening within them as a person. The cancer is not a separate, external entity and therefore ‘it’ cannot be ‘aggressive’ (as compared with an external infectious organism which could be described as aggressive). In the world of oncology, cancer is treated almost like a separate, external entity to be removed, poisoned or irradiated. This type of thinking may have evolved from the world of infectious diseases where microbes were the infectious agent, the enemy to be gotten rid of. The language that has evolved around this still positions cancer as something separate and unfortunately, the focus of many oncologists is on the disease, the tumor, the cells and not the human being.
Opening up the Conversation About the Patient’s Life Stresses
To open up the conversation about stresses in the patient’s life, Professor Sali typically begins with handing the patient a diagram such as the one in Fig. 2.1 that sets out in diagram form the relationship between the mind/stress/depression and the immune system, the endocrine system and the gut microbiome. He explains the different components involved in the stress response, including the link between the mind and storage of emotions and stress and the link between the mind/depression/nervous system and the immune system, endocrine system and the gut. Professor Sali elaborates on the concept of storage of emotions and stress, and how one can, by relieving some of the stresses in one’s life, positively influence health (via those afore-mentioned interdependent systems).
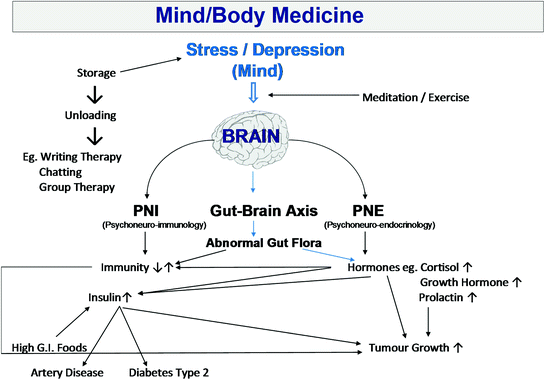

Fig. 2.1
Mind/body medicine
Talking through this diagram can be an ice-breaker—a means of opening up a discussion with the patient about their life stresses. It is most important that patients with cancer understand how stress negatively impacts on them, and come to identify what stresses might be present in their own lives. If the patient can understand what stresses may have contributed to their deviation from wellness, they may be in a better position to make the necessary changes to alleviate their stresses that will then contribute to positive changes in terms of the various bodily systems involved. As there is some evidence of traumatic events preceding a diagnosis of cancer, it is worthwhile talking with the patient about whether there have been any major events such as a death of a loved one.
Listening to the Patient’s Story
At this point, the clinician needs to sit back and listen, and simply prompt the patient to consider and talk about what difficulties they might be facing. This part of the consultation is critical, and in many ways facilitates a feeling, by the patient, of being heard and understood. Therefore, do not rush this part of the consultation—there is plenty of time for you to impart your knowledge. Listen carefully and you will be able to pick up important clues.
In discussing the link between mind/emotions and the body, it is important that the patient is not left feeling in some way guilty or anxious that they have somehow brought the cancer on themselves or ‘drawn’ cancer to them, for example, through negative thinking or actions. This is simply neither the case nor helpful to the patient. The power of the mind to positively impact on various pathophysiological pathways should be emphasised, as should how they can be proactive in changing the status quo—this is about empowering positive change through the power of the mind.
The discussion on storage of stress and emotions then leads onto a discussion on how the patient can help themselves by decreasing the storage of stress and emotions: by ‘unloading storage’.
Probing About Social Support
Unloading of stress and emotions is important in the prevention of illness, including cancer, and as part of a therapeutic approach. Social support and its role in unloading are extremely important. The disadvantages of poor social support have been discussed in the previous section. Therefore, the clinician should probe whether the cancer patient has social avenues for unloading in their personal life. If not, then there are professional options including working with a psychologist, cancer support groups, and other therapists. And there are other ways to unload stress and emotions, discussed later in this chapter.
Things to Look Out for
The conversation about stress is not a one-off conversation. It should be an ongoing dialogue over subsequent conversations, and an opportunity to check in with how the patient is doing.
Some things to look out for in consultations include symptoms and signs of:
Anxiety
Depression
Cancer-Related Fatigue.
During the initial and subsequent consultations, the clinician will need to be sensitive to the level of anxiety that the patient is likely to be experiencing, particularly on initial diagnosis but also at other time points. For example, following surgery, chemotherapy or radiation therapy, a patient will likely be extremely anxious about follow-up pathology results. Where results are not positive, or where the symptoms and signs associated with the cancer or cancer treatment are impacting physically on the patient, anxiety and depression can compound an already extremely difficult experience.
As we learned earlier, depression can also influence lifestyle factors that may negatively impact on health including smoking, consuming an excess of alcohol, lack of physical activity and excess weight [45]. So it is good to question the patient about their smoking status/habits, alcohol consumption and other lifestyle factors and gently probe to see if they are leaning on any of these as a crutch; lack of or a decrease in physical activity and increase in weight gain in someone who has previously indicated they were active may give some clues as to their current state of mind.
Assist the Patient to Explore Treatment Options for Anxiety and Depression
An integrative medicine approach will encourage the patient to explore various treatment options to help them with their anxiety and depression. For example, a timely referral to a psychologist or counsellor or a cancer support group may assist the patient work through and cope with anxiety and/or depression. At the very least, how the experience of cancer is impacting on the patient emotionally should be discussed with the patient at each consultation. The emotional aspects of what the patient with cancer and their loved ones are facing are critical to address, as the patient has much to gain if they can use their mind positively to impact their health. There is enough research to demonstrate how anxiety and depression can negatively impact on the body, creating in some ways a negative spiral.
Key Points
Be vigilant at all consultations for symptoms and signs of anxiety, depression and cancer-related fatigue
Refer the patient to an appropriate professional or support group for help to address the anxiety and depression.
Talking of Happiness
As humans, we have many things in common. One is that most humans seek happiness. It is an important goal to pursue. The fourteenth Dalai Lama says:
Whether one believes in religion or not, whether one believes in this religion or that religion, the very purpose of our life is happiness, the very motion of our life is toward happiness (His Holiness the Dalai Lama and Cutler [21]).Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree