8 The Role of Radiation Therapy in Early-Stage Supraglottic Cancer
Abstract
Early-stage supraglottic squamous cell carcinoma (ES-SSCC) has access to a relatively rich lymphatic drainage and therefore associated with an elevated risk of occult cervical lymph node involvement, even in clinically node negative patients. ES-SSCC is typically managed with larynx-preserving strategies, which include transoral laser microsurgery/robotic surgery, radiotherapy ± chemotherapy, or open supraglottic or suprahyoid laryngectomy. With appropriate selection, these modalities yield similar outcomes, with reported local control between 70 and 100% for stage T1N0M0 and 60 and 90% for stage T2N0M0. In addition to cancer cure rate, the choice of treatment modality is also based on anticipated speech and swallowing function, airway protection, patient choice, and institutional expertise. Comprehensive pretreatment assessment by a multidisciplinary team including, at the minimum, head and neck surgery, radiation oncology, medical oncology, and speech language pathology is critical. In patients with larger or unfavorable cancers treated with radiotherapy alone, intensification with accelerated or hyperfractionated radiotherapy is recommended as these altered fractionation regimens have been associated with improved locoregional control. Speech and swallowing rehabilitation, nutritional follow-up, and referral to a smoking cessation program should be carried out for optimal functional and quality-of-life outcomes.
8.1 Case Report
A 76-year-old Caucasian female, retired nurse aid, presented with a 3-month history of odynophagia and involuntary weight loss of 10 pounds. She was an active cigarette smoker, with a 30 pack-year history and drank alcohol socially. Her past medical history was relevant for chronic obstructive pulmonary disease (COPD), hypertension, osteoporosis, and previous hysterectomy for uterine fibroids. Her medications included an antihypertensive and vitamins.
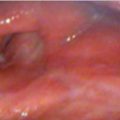
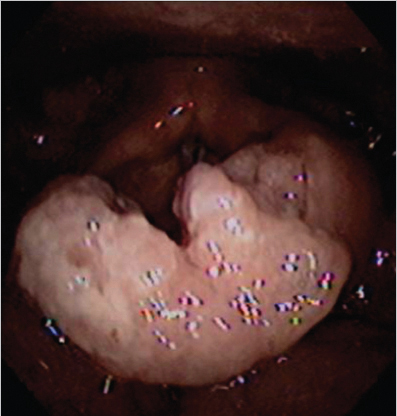
On physical examination, she had an Eastern Cooperative Oncology Group (ECOG) performance status of 1 and no palpable cervical or supraclavicular adenopathy. Examination of the oral cavity and oropharynx was unremarkable. Fiberoptic nasopharyngolaryngoscopy demonstrated obvious thickening of the epiglottis, with a friable exophytic mass involving the supra- and infrahyoid epiglottis, with the epicenter at the left laryngeal surface of the epiglottis. The cancer involved and expanded the left aryepiglottic fold without definite involvement of the left arytenoid or pyriform sinus. The vocal cords were free of cancer and fully mobile. There was no involvement of the base of the tongue or pharyngeal walls ( Fig. 8‑1).
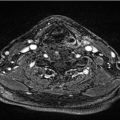
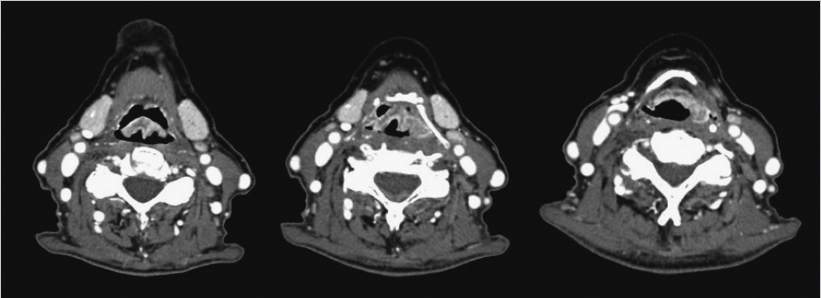
The patient had a contrast-enhanced computed tomography (CT) of the neck, which revealed an enhancing mass at the epiglottis (maximal thickness of 7 mm) over a length of 3 cm craniocaudally, with extension to the left aryepiglottic fold, and presence of nonspecific bilateral cervical lymph nodes ( Fig. 8‑2). She underwent direct microsuspension laryngoscopy with a biopsy of the supraglottic mass and rigid esophagoscopy. The biopsy revealed moderately to poorly differentiated invasive squamous carcinoma, p16 negative on immunohistochemistry. Fluorodeoxyglucose positron emission tomography (PET) /CT confirmed the primary extent and showed no hypermetabolic adenopathy. Chest images revealed findings consistent with COPD. There were no distant metastases. As the cancer involved more than one subsite of the supraglottis, had normal laryngeal/vocal fold mobility, and no signs of pre-epiglottic or extralaryngeal spread, final clinical stage was T2N0M0, American Joint Committee on Cancer (AJCC) group II (as per the eighth edition of the AJCC). 1 Upon interview, she reported mild dysphagia and odynophagia, but denied any signs or symptoms of aspiration. Evaluation with a speech-language pathologist and modified barium swallow showed mild dysphagia but no penetration or aspiration.
Following evaluation by head and neck surgery, and medical and radiation oncology, the patient’s case was discussed at the multidisciplinary tumor board. Considering her good swallowing function and adequate airway protection, tumor location, extent, and stage as well as patient factors such as older age and history of COPD, definitive radiation therapy was recommended. Concurrent chemotherapy was considered, but given her age, final recommendations were for altered fractionation radiation therapy alone. In addition, the patient was referred to a smoking cessation program. At the time of radiotherapy simulation, the patient was positioned in a supine position, immobilized with a custom head, neck, and shoulder thermoplastic mask, to ensure reproducibility of the patient’s position. CT images were obtained at 2 -mm slice thickness for treatment planning purposes. To reduce internal cancer/laryngeal motion, the patient was given specific instruction for quiet respiration and not to swallow during simulation, image acquisition, or treatment delivery.
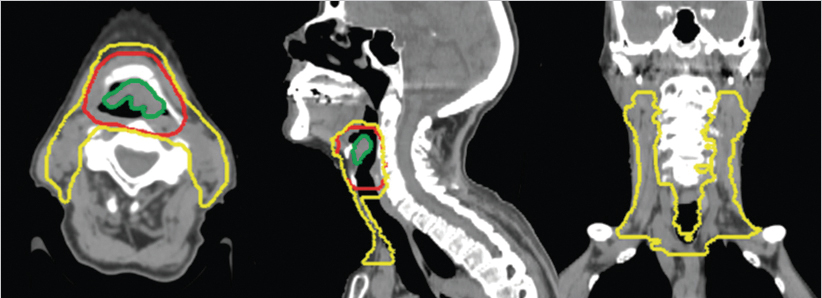
The radiation therapy target volumes were as follows: (1) a high-risk target volume encompassing the gross tumor volume (defined by combination of endoscopic and diagnostic imaging findings) plus an 8-mm margin as well as the adjacent larynx at risk in order to account for internal laryngeal motion (largely in the craniocaudal direction) and (2) a standard risk or elective target volume encompassing the bilateral cervical lymph nodes at risk for harboring microscopic regional disease, namely, lymph node levels II to IV bilaterally, the most cranial extent being the subdigastric nodes ( Fig. 8‑3). A modified Danish Head and Neck Cancer Group (DAHANCA) fractionation schedule was administered, with a dose of 70 Gy to the high-risk target volume and 57 Gy to the standard-risk target volume, given in 35 fractions over 6 weeks, with 6 fractions per week (BID one day per week with ≥ 6 hours between fractions) for 5 of the 6 weeks. Treatment was planned and delivered using volumetric-modulated arc therapy (VMAT), with two full arcs, each with slight collimator rotation. The treatment plan was optimized to cover the aforementioned targets while respecting spinal cord tolerance and constraining as feasible the bilateral parotid glands, bilateral submandibular glands, oral cavity, lungs, shoulders, and cervical esophagus.
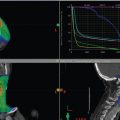
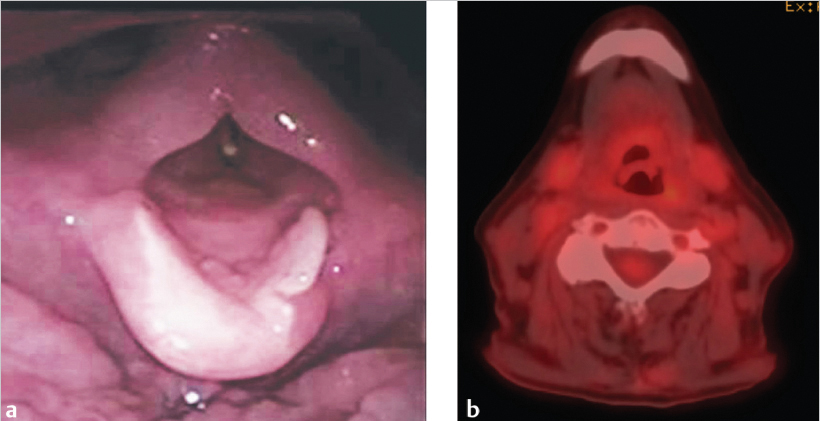
The patient tolerated the treatment well and completed it without interruption, hospitalization, or feeding tube placement. Acute toxicities included grade 2 dermatitis and grade 3 mucositis for which she required temporary use of narcotics. Contrast-enhanced CT of the neck and videoendoscopy 2 months after completion of radiation therapy showed resolution of the supraglottic cancer with treatment effects, namely, edema with anatomic distortion ( Fig. 8‑4 a). PET/CT carried out 3 months after the completion of treatment demonstrated no residual metabolic activity ( Fig. 8‑4 b). The patient remains disease free and able to eat a regular solid diet now 3 years after treatment.
8.2 Discussion
Early-stage supraglottic squamous cell carcinoma (ES-SSCC) generally includes stage I (T1N0M0) or stage II (T2N0M0) and perhaps those with N1 disease. While T1 cancers are limited to one subsite of the supraglottis with normal vocal cord mobility, T2 cancers invade the mucosa of more than one adjacent subsite of the supraglottis or have impaired vocal cord mobility, without laryngeal fixation or pre-epiglottic invasion. Due to the richness of draining lymphatics of the supraglottis, node-negative ES-SSCC may have up to a 40% risk microscopic nodal involvement. 2 Treatment strategies generally seek to address this risk for microscopic disease (e.g., elective nodal irradiation) and compared to the excellent outcomes of their early-stage glottic cancer counterpart, the 5-year overall survival for ES-SSCC is generally below 70%. 3 Treatment strategies for ES-SSCC are typically single modality, based on organ preservation and focus on maximizing disease control and survival while maintaining good laryngeal function (e.g., swallowing, airway protection, and vocal quality) and patient’s quality of life. 4 , 5 Treatment options for ES-SSCC include (1) transoral laser microsurgery, transoral robotic surgery, or open supraglottic or suprahyoid laryngectomy (selected cases) ± neck dissection ± adjuvant radiotherapy 6 , 7 , 8 , 9 or (2) definitive radiotherapy alone, or in combination with systemic therapy for selected more unfavorable cancers with goals to improve local control and larynx preservation probability. 10 , 11 , 12 These treatment approaches yield local control between 70 and 100% for stage I and 60 and 90% for stage II. 9 , 10 , 12 , 13 , 14 , 15 , 16 Table 8‑1 presents some published institutional outcomes for definitive radiotherapy in ES-SSCC. Retrospective studies, systematic reviews, and meta-analysis have compared outcomes of organ-preserving surgery versus radiotherapy, but conclusions have been inconsistent. 17 , 18 , 19 , 20 In the context of the inherent selection biases associated with retrospective comparisons and in the absence of randomized study, the optimal treatment strategy for ES-SSCC remains a subject of debate.
As per the most recent American Society of Clinical Oncology Clinical Practice Guidelines, both organ-preserving surgery and radiotherapy offer larynx preservation potential without compromising survival, and optimal management of ES-SSCC should be selected individually after careful consideration of patient and cancers characteristics, patient choice, and institutional expertise. Treatment decision should involve multidisciplinary evaluation and thorough physician-to-patient discussions eliciting personal preference, considering the potential advantages and trade-offs between approaches. As a general rule, most T1N0 tumors and nonbulky T2N0 cancers can be effectively managed with definitive radiotherapy as a single modality. Patient’s characteristics, such as the presence of major medical problems precluding the use of general anesthesia, inadequate pulmonary reserve such as the presence of COPD, advanced age, or judged elevated risk of aspiration after supraglottic laryngectomy are factors in favor of a radiotherapy-based approach. 21 In addition, involvement of the vocal cords, anterior commissure, or bilateral arytenoids can preclude adequate organ-preserving surgery. 22 Organ-preserving surgery is generally favored for younger patients when feasible. 23 Indications for postoperative radiotherapy include close/positive margins, presence of perineural invasion or lymphovascular invasion, 24 or incidental upstaging features (e.g., multiple nodes, extranodal extension).
For patients with ES-SSCC with an unfavorable or bulky cancer to be treated with radiotherapy alone, treatment intensification through altered fractionation, namely, accelerated or hyperfractionated radiotherapy, is favored to improve local control. 25 Standard fractionation is defined as administration of approximately 2 Gy per daily fraction, 5 fractions per week, over 6.5 to 7 weeks, to a total dose of 66 to 70 Gy. Altered fractionation is accomplished by either shortening the overall treatment time, escalating the total dose, or sometimes both, and is generally achieved by delivering multiple smaller fractions per day for some of or the entire treatment course. Radiotherapy regimens vary between institutions; common altered fractionation schedules include the following:
Modified, moderately accelerated DAHANCA fractionation of 66 to 70 Gy in 33 to 35 fractions of 2 Gy each delivered over 6 weeks; 6 fractions a week (twice a day once per week with ≥6 hours between fractions), delivered over a total of 6 weeks.
Accelerated fractionation using concomitant boost, administering a total of 72 Gy in 42 fractions with an initial plan of 54 Gy in 30 fractions and a second plan focused on the high-risk volume of 18 Gy in 12 fractions of 1.5 Gy. The second plan is administered concurrently with the first plan over the last 12 days of treatment, given as a second daily fraction, with ≥6 hours between fractions.
Hyperfractionated regimen of 76.8 to 79.2 Gy in 64 to 66 fractions of 1.2 Gy, administered twice a day with ≥6 hours between fractions.
A meta-analysis of 15 trials, involving 6,515 patients treated with head and neck radiotherapy, including 34% primary cancer of the larynx, revealed a 3.4 and 6.4% absolute benefit in 5-year overall survival and locoregional control, respectively, with altered fractionation versus standard fractionation. 25 The absolute overall survival benefit was found higher with hyperfractionation versus accelerated radiotherapy (8 vs. 2%). Additionally, when medically fit, patients with an unfavorable or deeply invasive T2N0 cancer are considered for concurrent platinum-based chemoradiation or sequential chemoradiation, in an attempt to further intensify treatment in patients felt to be at higher risk of poorer disease control. 12 , 26 When given concomitantly with chemotherapy, the standard radiotherapy fractionation schedule is generally used.
Intensity-modulated radiotherapy (IMRT) is the preferred technique for the delivery of locoregional radiotherapy to the head and neck. In fact, compared to 3D conformal radiotherapy, IMRT allows for relative sparing of many normal organs, particularly the parotid glands. In a randomized trial, IMRT was shown to reduce the rates of grade ≥ 2 xerostomia by 50% at 1-year postradiotherapy. 27 VMAT is a contemporary form of IMRT, where variable gantry speed, dose rate, and dynamic multileaf collimation are used, which significantly shorten radiation delivery time. 28 VMAT can therefore be specifically advantageous in the treatment of cancer of the larnyx where swallowing motion is a concern. Likewise, intensity-modulated proton therapy is another advanced form of highly conformal radiotherapy with the potential to further improve patient outcomes through the elimination of unnecessary radiation to surrounding nontarget tissues.
For optimal functional and quality-of-life outcomes as well as for airway protection, assessment of speech, swallowing, and nutritional status should be carried out at baseline, during and after radiotherapy. Speech and swallowing rehabilitation, including early implementation of prophylactic exercises and swallowing maneuvers teaching (a.k.a. “eat and exercise”), is crucial for accelerated and full posttreatment recovery. 29 , 30 In addition, patients with supraglottic cancers receiving voice rehabilitation were shown to have improvements in voice outcomes after therapy. 31 An expert dietician should closely monitor the patient’s body weight, nutritional needs, and hydration throughout treatment and after treatment to reduce the loss of weight and lean body mass, minimize treatment delays, and hospitalization rates, and to improve treatment outcomes. 32
As per the National Comprehensive Cancer Network guidelines version 2.2017, surveillance after treatment completion should include history, physical examination, and fiberoptic nasopharyngolaryngoscopy every 1 to 3 months for the first year, every 2 to 6 months for the second year, every 6 months for the third to fifth year, and annually thereafter. A CT of the neck is typically obtained at 2 months after treatment, and further imaging as indicated in the presence of worrisome signs or symptoms, although ongoing surveillance neck imaging is routine at many centers. With rates of hypothyroidism after head and neck irradiation being around 30%, 33 routine thyroid-stimulating hormone levels should be obtained at every visit or at least every 6 months and thyroid hormone replacement should be prescribed for patients who are clinically hypothyroid. Chest imaging should be considered in patients with a significant history of smoking for detection of second primary cancers. Adequate speech and swallowing therapy and rehabilitation should be pursued during the follow-up period.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree