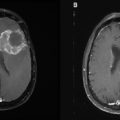
Radioimmunotherapy in Lymphomas
Rush University Medical Center/Rush University, Chicago, IL, USA
Radioimmunotherapy (RIT) combines a radiation-emitting radionuclide with an anti-CD20 monoclonal antibody to treat B-cell non-Hodgkin’s lymphoma (NHL). The two approved agents are 90Y-ibritumomab tiuxetan and 131I-tositumomab. RIT is approved for treatment of patients with relapsed or refractory follicular lymphoma, including patients with rituximab refractory disease.
There are some differences in the approvals between the two agents. The label for 131I-tositumomab also includes patients with transformed lymphomas, and 90Y-ibritumomab tiuxetan was approved in 2009 for use as consolidation therapy after induction chemotherapy for patients with follicular lymphoma who achieve a partial or complete remission after first-line chemotherapy.
RIT studies demonstrate favorable efficacy and safety profiles in follicular lymphoma with the primary toxicity being reversible myelosuppression. Many studies have demonstrated higher overall response rates and durations of response when used earlier in the treatment algorithm for follicular or indolent lymphoma. This chapter asks a number of questions that will drive home the nuances of RIT use.
Multiple Choice Questions
1. A patient presents in consultation with relapsed follicular lymphoma to discuss RIT. Which of the following criteria would exclude the patient from receiving RIT?
- Greater than 25% bone marrow involvement
- Platelet count of ≥100,000/mm3
- Absolute neutrophil count of 1800/mm3
- Prior stem cell transplant
Prior to receiving RIT, adequate hematopoietic reserve must be established as myelosuppression is clearly the dose-limiting toxicity. As such, recommendations are that the marrow should have less than 25% lymphoma burden with normal cellularity of at least 15%, and peripheral blood counts should demonstrate an absolute neutrophil count of ≥1500 × 106/L and a platelet count ≥100,000 × 106/L. 90Y-ibritumomab tiuxetan is dosed at 0.4 mCi/kg for a pretreatment platelet count of at least 150,000/mm3 and 0.3 mCi/kg for a platelet count ranging between 100,000/mm3 and 149,000/mm3. Similarly, impaired marrow reserve is also assumed in those who have received external beam radiation to more than 25% of the marrow or with a history of failed stem cell transplant. Although prior high-dose therapy has been a contraindication to RIT in the past, it is now felt that reduced-dose RIT can be administered safely in patients treated with prior high-dose therapy with stem cell support provided there is adequate marrow reserve. Candidates should not be restricted on the basis of a high-risk clinical presentation—patients with high International Prognostic Index (IPI), extranodal involvement, or chemoresistance have been shown to derive benefit from RIT. Reasonable response rates have also been reported in patients with high tumor burden, including bulky disease, although these rates are not as high (68%). In previously treated patients, caution should be exercised to exclude a preexisting myelodysplastic syndrome (MDS) with fluorescence in situ hybridization (FISH) or conventional cytogenetics prior to RIT therapy.
2. A female patient with newly diagnosed follicular lymphoma asks you what the differences are between 90Y-ibritumomab tiuxetan and 131I-tositumomab. She is concerned about what the implications are for her child as she is a single mother. You indicate:
- Ibritumomab is better than tositumomab.
- Ibritumomab is a beta emitter while tositumomab is a gamma emitter, making it preferred to isolate patients receiving tositumomab from children for a short period of time after dosing.
- Long-term side effects of ibritumomab include hypothyroidism.
- The bioscan is a good predictor for altered ibritumomab biodistribution and should always be used.
- Long-term side effects of ibritumomab include hypothyroidism.
With a newly diagnosed follicular lymphoma, this patient is not eligible for RIT monotherapy. Please see indications for use of these agents as stated in the introduction. Chemoimmunotherapy induction followed by RIT consolidation is, however, a reasonable option. 131I-tositumomab (BEXXAR®, GlaxoSmithKline) and 90Y-ibritumomab tiuxetan (Zevalin®, Spectrum Pharmaceuticals, Inc.) are the only two US Food and Drug Administration (FDA)-approved radioimmunotherapeutic agents currently available in the United States for indolent lymphomas. 131I-tositumomab is a gamma emitter composed of a murine IgG2a λ anti-CD20 antibody conjugated to 131I. Clinical trials with 131I-tositumomab predate those with 90Y-ibritumomab tiuxetan, allowing for more reliable long-term follow-up and understanding of toxicities. Due to the nature of its emissions, radiation safety, including keeping away from children and pregnant women, should be followed for 1 week after a therapeutic dose, a precaution that has resulted in its falling out of favor. Other side effects of 131I-tositumomab include hypothyroidism and myelosuppression. 90Y-ibritumomab tiuxetan, however, is a beta emitter with minimal risk of exposure. Data comparing the efficacy of 90Y versus 131I in therapeutic effect are conflicting. Of note, analysis of data in 253 patients showed that the 111In imaging dose and bioscan were not reliable predictors of altered 90Y biodistribution. Thus, the requirement for a bioscan for 90Y has been removed by the FDA.
3. A 59-year-old male was diagnosed with follicular lymphoma 6 years ago after presenting with bulky lymphadenopathy and B-symptoms to his primary care provider. The decision was made to treat this patient with R-FND (rituximab, fludarabine, mitoxantrone, and dexamethasone) for six cycles, the patient achieving a CR. However, 3 years later, the patient relapsed, and this time he underwent retreatment with R-FND followed by RIT consolidation. Today he presents with fatigue, dyspnea on exertion, and petecchiae. Lab work demonstrates pancytopenia. This patient likely has:
- Therapy-related MDS
- Relapsed follicular lymphoma
- Transformed follicular lymphoma
- Active infection
RIT conjugates are relatively new on the market, making information on long-term sequelae limited. Concern for an increased propensity for developing therapy-related myelodysplastic syndrome (t-MDS) and/or acute myeloid leukemia (AML) with RIT has been raised. However, to date, this has not been verified. In retrospective analyses of relapsed or refractory indolent lymphoma patients receiving RIT, 2.5–3.0% cases of MDS or AML have been reported. This incidence is no different from that described in patients receiving alternative therapies for their lymphoma with an annualized rate of 0.7% per year in RIT patients compared to an annualized rate of 1.0–1.5% per year after treatment with alkylating agents. There is some suspicion that prior exposure to purine nucleoside analogs may be compounding this effect by further damaging the stem cell environment. More recently, in an update of the prospective phase III First-Line Indolent Trial (FIT) using RIT consolidation in the front-line setting, no difference in rates of MDS and AML cases were seen in the 90Y-ibritumomab versus the control arm (3% versus 1%, P = 0.063) with 66.2 months of follow-up. However, prolonged cytopenias were seen with patients receiving induction with fludarabine-based regimens as compared to other chemotherapeutic agents.
4. A 54-year-old female presents with a new diagnosis of follicular lymphoma. She asks about her options for therapy and has been entertaining the idea of RIT. Which of the following are false regarding RIT?