5 Hepatocellular Carcinoma: Chemoembolization
5.1 Introduction
Patients with hepatocellular carcinoma (HCC) frequently present in later stages when curative treatments are no longer an option. 1 Therefore, the majority of patients with HCC eligible for treatment will undergo noncurative treatments, of which transarterial chemoembolization is the most commonly performed procedure. Chemoembolization is defined as the infusion of a mixture of chemotherapeutic agents with or without ethiodized oil followed by embolization with particles such as polyvinyl alcohol, calibrated microspheres, or Gelfoam. 2 Variation in patient selection and procedure technique among institutions has led to significant heterogeneity in response and survival, making uniform treatment recommendations challenging.
HCC has been treated with transcatheter embolization since the late 1970s. 3 Many early reports regarding chemoembolization reported mixed results. However, the publication of two randomized trials in 2002 established chemoembolization as standard of care for patients with unresectable HCC. 4 , 5 The ensuing widespread use and proven results of chemoembolization have resulted in its incorporation into standard treatment guidelines for HCC (Barcelona Clinic Liver Cancer, National Comprehensive Cancer Network).
Depending on the techniques employed, tumor death is caused by the cytotoxic effects through achieving high intratumoral concentration of chemotherapy, the ischemia induced by embolization, or both. In the case of oil-based chemoembolization, the mechanism of action relates to both the cytotoxic effects of the chemotherapy and the ischemic effects induced by embolization. Embolization also prevents washout of the chemotherapeutic agent into the systemic circulation. In contrast, drug-eluting bead (DEB) chemoembolization may have a different mechanism of action. Animal models suggest that tumor necrosis in DEB chemoembolization is related to the high concentration of doxorubicin eluting from the beads rather than the mechanical effects of cessation of blood flow, as with oil-based chemoembolization and bland embolization. 6
5.2 Indications
Chemoembolization is generally considered the first-line noncurative therapy for patients with early- and intermediate-stage HCC. Chemoembolization is not typically performed in patients with a curative treatment option, which includes surgical resection, thermal ablation, and liver transplantation. However, at some centers where transplant waiting lists are long, chemoembolization can be used as a “bridge to transplant.” In these patients the procedure is performed to prevent disease progression beyond Milan criteria (single tumor ≤ 5 or three tumors ≤ 3 cm). Patients undergoing chemoembolization should have adequate hepatic function (Child–Pugh class A or B) and acceptable functional performance status (Eastern Cooperative Oncology Group [ECOG] 0–2). Chemoembolization can also be done in conjunction with an ablation procedure in intermediate-sized HCC (3–5 cm). 7 It is also the most common therapy employed for recurrent disease.
5.3 Contraindications
Chemoembolization has typically been contraindicated in patients with advanced liver disease (Child–Pugh class C). Low serum albumin, elevated bilirubin, and encephalopathy are independent predictors of poor tolerability to chemoembolization. Although a total bilirubin > 3 mg/dL is considered a contraindication to chemoembolization due to the high risk of permanent liver failure, exceptions can be considered when performing a superselective chemoembolization or if performing a chemoembolization as a bridge to transplantation. 8
Chemoembolization is also contraindicated in patients with poor functional status (ECOG performance status > 2) and in patients with extrahepatic disease. The presence of vascular invasion is also considered a relative contraindication because of the increased risk of liver failure and the poor overall tumor response and survival of these patients. 8 However, depending on the degree of invasion and other patient factors, there is some evidence that it can be performed safely and with mild potential benefit. 9 Other relative contraindications include uncorrectable coagulopathy, severe renal insufficiency, a previous procedure causing biliary incompetence (e.g., biliary stent placement, prior Whipple procedure), and leukopenia.
5.4 Patient Selection and Preprocedure Workup
Ideally, patients should undergo evaluation by a multidisciplinary group consisting of members from transplant surgery, hepatobiliary surgery, interventional radiology, medical oncology, and hepatology/gastroenterology departments. A curative procedure should not be an option, although the merits of ablation versus chemoembolization in the setting of transplant candidates are controversial and institution dependent. All patients should undergo a complete history and physical with particular attention to liver disease and the patients’ overall health or performance status. A recent multiphase magnetic resonance imaging (MRI) or computed tomographic (CT) scan prior to chemoembolization is required in order to properly stage the patient and to plan therapy. Recent laboratory values, including liver function tests, complete blood count, creatinine, coagulation profile, and nonmaternal alpha-fetoprotein should be obtained both for accurate staging and to confirm that the status has not changed before the procedure. It is not uncommon for these patients to have thrombocytopenia or fluctuating liver function. An evaluation of cardiac function, such as with echocardiography or a multigated acquisition (MUGA) scan, should be considered in patients with known heart disease because doxorubicin can be cardiotoxic and is contraindicated in patients with congestive heart failure.
5.5 Technique
Antibiotic prophylaxis is normally provided for appropriate coverage of skin and gastrointestinal flora (i.e., 1 g cefazolin and 500 mg metronidazole). Procedures are performed under moderate sedation, typically using intravenous midazolam and fentanyl. Patients are also often treated prophylactically for postembolization syndrome, usually with antiemetics such as 5-HT3 (serotonin) receptor antagonists (e.g., palonosetron, ondansetron).
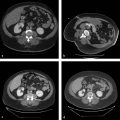
On the initial round of chemoembolization, a thorough angiographic evaluation of the hepatic arterial circulation should be done. Even when performing chemoembolization for a single focal tumor, multiple tumors can be identified on angiography that were previously not seen on baseline cross-sectional imaging. Furthermore, feeding arteries to the tumor may arise from unexpected origins (e.g., segment VIII tumor being fed by the left hepatic artery). Access into the common femoral artery is obtained using a standard technique, with insertion of a 4 to 6 French sheath. Selection and digital subtraction angiography (DSA) of the superior mesenteric artery should be performed in order to evaluate potential variant hepatic anatomy (e.g., replaced or accessory right hepatic artery) and to assess for portal venous flow on delayed imaging. Catheterization is typically performed with a standard 4 or 5 French diagnostic catheter. Commonly used catheters include the Simmons 1, Sos, Cobra 2, and RC2. Subsequently, catheterization and DSA of the celiac trunk are done ( Fig. 5.1 ). During these initial steps, reference to the arterial phase of the patient’s baseline cross-sectional imaging will expedite successful catheterization. In cases where preprocedural arterial imaging is inadequate, a flush aortogram can delineate the mesenteric anatomy in order to guide the choice of base catheter for proper catheterization.
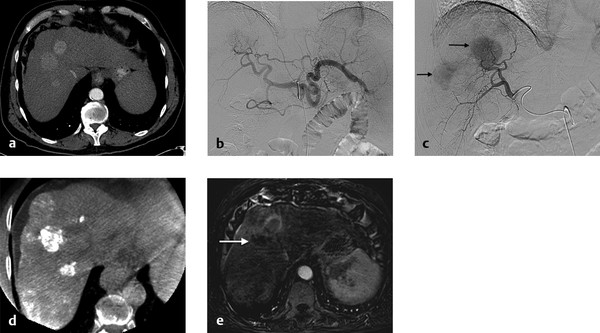
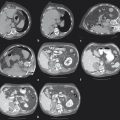
Catheterization of the common or proper hepatic artery should be done with a microcatheter (e.g., 2.8 French distal outer diameter, Progreat, Terumo Medical Corporation, or Renegade Hi-Flo, Boston Scientific) using a range of 0.014 to 0.021 wires. Then selective catheterization and DSA of both the right and left hepatic arteries are performed. Chemoembolization should be performed selectively whenever possible ( Fig. 5.2 ). In diminutive-caliber vessels, a smaller microcatheter in conjunction with softer 0.014 wires should be considered (e.g., 2.3 French distal outer diameter, Prowler Plus, Codman, or Maestro, Merit). If there are multiple feeding arteries to a tumor or if there are multiple tumors in a single lobe, treatment should be performed proximally and/or in a lobar fashion in order to cover the entire perfused territory ( Fig. 5.1 ).

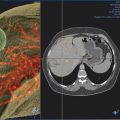
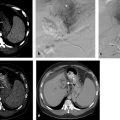
The additional use of intraprocedural cone-beam CT has gained increasing acceptance. 10 This is typically performed with contrast injection through the microcatheter positioned in the proper or lobar hepatic arteries. Contrast should be diluted, and the injection should be performed throughout the duration of the CT acquisition, which is typically acquired 4 to 6 s after the start of contrast injection. This will allow for arterial phase imaging, which helps to detect all feeding arteries to the tumor as well as a parenchymal phase in order to assess the number, location, and size of the hypervascular tumors.
Chemotherapy and embolic combinations vary considerably. The lack of standardization is in part due to the paucity of available randomized literature comparing different treatment specifics. 11 As a result, no single chemoembolic platform is the standard. Therefore, the most common formulation used in the United States will be discussed. Oil-based chemoembolization has been traditionally done. Multiple agents can be combined, such as cisplatin, mitomycin-C, and doxorubicin; however, the limited availability of some of these agents has resulted in many centers now using a single agent. This consists of mixing 50 to 100 mg doxorubicin with 10 to 20 mL ethiodized oil (Guebert, Indiana). Although no survival benefit exists for systemic doxorubicin in the setting of HCC, localized intra-arterial delivery allowing high intratumoral and low systemic concentrations of the drug can result in significant tumor response and prolonged survival. The oil acts as an emulsifying agent and a lipophilic carrier in order to allow localized drug delivery without rapid washout. Infusion is performed under live fluoroscopic guidance to prevent reflux of chemotherapy and assess for arterial stasis. When infusing, complete stasis should be avoided because it will prevent future repeat treatments if necessary. After infusion of chemotherapy, follow-up embolization is performed. Gelatin slurry (Pfizer), embospheres (Merit), and PVA (Boston Scientific) are the most commonly used embolic agents. Again, complete stasis should be avoided in order to allow for repeat treatments. Furthermore, it has been shown that achieving stasis does not result in better response or better patient outcomes. 12
Embolization with DEB has gained popularity over traditional oil-based chemoembolization. Due to the prolonged binding properties of DEBs with doxorubicin, the drug is slowly released into the tumor, reaching higher local concentration and decreased systemic concentration when compared to oil-based chemoembolization. This may allow for decreased side effects and improved tolerance in some patients. 13 The most common formulation is to combine 50 to 150 mg doxorubicin with 100 to 300 µm particles (LC Beads, Biocompatibles, Inc.). 14 A 50 to 75 mg doxorubicin vial is reconstituted with 2 mL of sterile water. The saline from the LC bead vial is removed. The reconstituted doxorubicin solution is directly added to the bead vial, and then 60 minutes are needed to allow the drug to bind to the beads. The total mixture is diluted with nonionic iodinated contrast (20–30 mL). Infusion should be done using intermittent fluoroscopy, with a slow injection rate. After delivery of the beads, postembolization angiography is performed. Similar to oil-based chemoembolization, arterial stasis should be avoided in order to allow for future repeated treatments.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree