Section 5 Chronic complications
Ocular complications
Clinically significant ocular complications associated with diabetes include:
• transient visual disturbances secondary to osmotic changes (or briefer episodes secondary to hypoglycaemia)
• retinopathy – the predominant cause of blindness in type 1 diabetes
• cataracts – develop earlier in diabetic patients
• glaucoma – primary or secondary to diabetic retinopathy
Diabetic retinopathy
Pre-proliferative diabetic retinopathy
Pre-proliferative diabetic retinopathy is characterized by:
• vascular changes consisting of venous changes in the form of ‘beading’, ‘looping’ and ‘sausage-like’ segmentation. The arterioles may also be narrowed and even obliterated, resembling a branch retinal artery occlusion.
• cotton-wool spots, caused by capillary occlusion in the retinal nerve fibre layer. Interruption of axoplasmic flow caused by the ischaemia, and subsequent build-up of transported material within the nerve axons, is responsible for the white appearance of these lesions.
• dark blot haemorrhages, which represent haemorrhagic retinal infarcts.
• intraretinal microvascular anomalies (IRMAs), representing either dilated pre-existing vessels or early intraretinal new vessels. They are found within an area of capillary non-perfusion.
Classification of diabetic retinopathy
Conventionally involvement of retina in patients with diabetes has been classified as background, pre-proliferative retinopathy and proliferative retinopathy. Macula may be involved (maculopathy) with any of the above forms of retinopathy (Table 5.1).
Table 5.1 Grading system used for screening for diabetic retinopathy (UK National Guidelines)
| RETINOPATHY (R) |
The presence of at least one of any of the following features anywhere: • Four or more blot haemorrhages in one hemi-field only (inferior and superior hemi-fields delineated by a line passing through the centre of the fovea and optic disc) Any of the following features: Any of the following features: |
| MACULOPATHY (M) |
| No features ≤ 2 disc diameters from the centre of the fovea sufficient to qualify for M1 or M2 as defined below. Level M1: Lesions as specified below within a radius of > 1 but ≤ 2 disc diameters from the centre of the fovea Level M2: Lesions as specified below within a radius of ≤ 1 disc diameter of the centre of the fovea |
| PHOTOCOAGULATION (P) |
| OTHER LESIONS (OL) |
| Other non-diabetic lesions present: |
IRMA, intraretinal microvascular anomaly.
Another commonly used grading system is the Early Treatment Diabetic Retinopathy Study (ETDRS) system (Table 5.2).
Table 5.2 The Early Treatment Diabetic Retinopathy Study (ETDRS) system
| Grade | Features |
|---|---|
| NPDR | |
| None | Normal retina |
| Early | Microaneurysms only |
| Mild | Microaneurysms plus: |
| Moderate | Mild; NPDR plus: |
| Severe | Moderate NPDR plus 4/2/1 rule: |
| Very severe | Any two or more of the ‘severe categories’ |
| PDR | |
| PDR without HRC | NVE or NVD < ½DD |
| PDR with HRC | NVE or NVD > ½DD plus preretinal and/or vitreous haemorrhages |
DD, disc diameter; HRC, high-risk changes; IRMA, intraretinal microvascular anomaly; NPDR, non-proliferative diabetic retinopathy; NVD, new vessels on the disc; NVE, new vessels elsewhere in the retina; PDR, proliferative diabetic retinopathy.
Screening
Grading and quality assurance
Automated grading can operate as the initial screener to exclude a majority of images with ‘no retinopathy’ before manual grading. The specificity of automated grading is lower than for manual grading, for equivalent sensitivity. Automated grading may be used for distinguishing no retinopathy from any retinopathy in a screening programme providing validated software is used. It has a similar sensitivity for detecting referable retinopathy, but may be less sensitive at detecting diabetic maculopathy (Table 5.3).
Table 5.3 Systematic screening for diabetic retinal disease
General principles of management of diabetic retinopathy
Proliferative diabetic retinopathy
Neovascularization is the hallmark of PDR. New vessels are commonly seen along the retinal arcades, but can occur at the optic disc or elsewhere in the retina. As a general rule, the retina distal to the neovascularization should be considered as ischaemic, and it has been estimated that more than one-quarter of the retina has to be non-perfused before neovascularization occurs. Ischaemia upregulates the vascular endothelial growth factor (VEGF) that stimulates neovascularization. New vessels start as endothelial proliferations, and pass through the internal limiting membrane to lie in the potential plane between the retina and the posterior vitreous face. PDR can result in visual deterioration from ischaemia, haemorrhage and tractional retinal detachment involving the macula (Table 5.4).
Table 5.4 Guidelines for referral for specialist assessment of diabetic retinopathy
| Clinical problem | Urgency (within) |
|---|---|
| Sudden loss of vision | 1 day |
| Evidence of retinal detachment | 1 day |
| New vessel formation (on the disc or elsewhere) | 1 week |
| Vitreous or pre-retinal haemorrhage | 1 week |
| Rubeosis iridis | 1 week |
| Hard exudates within 1DD of the fovea or clinically significant macular oedema | 4 weeks |
| Unexplained drop in visual acuity | 4 weeks |
| Unexplained retinal findings | 4 weeks |
| Severe or very severe non-proliferative retinopathy is present | 4 weeks |
Diabetic maculopathy
Diabetic maculopathy can present in the following patterns:
• Focal – focal leakage, characterized usually by the presence of a cluster of microaneurysms surrounded by retinal thickening and a complete or incomplete ring of hard exudates
• Diffuse – diffuse retinal thickening and exudation from capillaries often involving the fovea. Cystoid changes suggest chronicity of the condition
• Ischaemic – diagnosed ideally by fluorescein angiography, representing an enlarged or irregular foveal avascular zone. Clinically it may lack features; however, the hallmarks are reduced visual acuity, deep blot haemorrhages and IRMAs.
Vitrectomy
• Patients with type 1 diabetes and persistent vitreous haemorrhage should be referred for early vitrectomy.
• Vitrectomy should be performed in patients with tractional retinal detachment threatening the macula and should be considered in patients with severe fibrovascular proliferation.
• Patients with type 2 diabetes and vitreous haemorrhage that is too severe to allow photocoagulation should be referred for consideration of a vitrectomy.
Cataract extractions in patients with diabetes
• Cataract extraction should not be delayed in patients with diabetes.
• Cataract extraction is advised when sight-threatening retinopathy cannot be excluded.
Diabetic neuropathy
Age
Diabetic neuropathy can manifest with a wide variety of sensory, motor and autonomic symptoms:
• Sensory symptoms may be negative or positive, diffuse or focal.
• Motor problems may include distal, proximal, or more focal weakness.
• The autonomic nervous system is composed of nerves serving the heart, skin, eyes, gastrointestinal system and genitourinary system. Autonomic neuropathy can affect any of these organ systems.
Classification of neuropathy
Symmetrical polyneuropathies
Symmetrical polyneuropathies involve multiple nerves diffusely and symmetrically:
• Distal symmetrical polyneuropathy:
• Diabetic autonomic neuropathy:
• Diabetic neuropathic cachexia:
Asymmetrical neuropathies
• Diabetic thoracic radiculoneuropathy:
• Diabetic radiculoplexus neuropathy:
Treatment
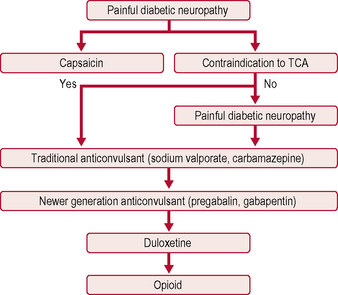
The only two drugs approved by the US Food and Drug Administration (FDA) for diabetic peripheral neuropathy are the antidepressant duloxetine and the anticonvulsant pregabalin (Fig. 5.1). Before trying a systemic medication, people with localized diabetic periperal neuropathy occasionally get relief from their symptoms with lidocaine patches.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree