33 Targeted Therapy for the Treatment of Advanced Squamous Cell Carcinoma of the Larynx
Abstract
In this chapter, systemic agents targeted against the epidermal growth factor receptor and the programed death 1: programed death ligand 1 pathway are discussed. The trials leading to the approval of cetuximab in the first-line setting and nivolumab after platinum failure are highlighted and exploration of predictive biomarkers and future directions are discussed.
33.1 Introduction
For patients with squamous cell carcinoma (SCC) of the larynx with a locoregional recurrence not amenable to surgery or radiation, and/or metastatic disease, palliative systemic therapy is the only option for treatment. Traditional cytotoxic chemotherapeutic agents used in SCC of the head and neck (HNSCC) include platinum (carboplatin, cisplatin), taxanes (paclitaxel, docetaxel), and 5-fluorouracil (5FU). In the recurrent/metastatic (R/M) setting, no platinum-based doublet has improved overall survival (OS) in non-nasopharyngeal HNSCC compared to a single agent, although doublets are associated with significantly higher response rates. 1 , 2 Platinum-based doublet combinations, cisplatin 5FU and cisplatin paclitaxel have been compared in a phase III trial with no difference in efficacy. 3 Outcomes with traditional chemotherapy regimens alone remain poor with median OS between 8 and 9 months. 3 In an effort to improve outcomes for patients with R/M HNSCC including laryngeal SCC, molecular pathways involved in oncogenesis and proliferation, as well as immune co-signaling have been targeted. In this chapter, targeted therapy will be discussed with a focus on the epidermal growth factor receptor (EGFR) and the programed death 1 (PD-1): programed death ligand 1 (PD-L1) pathway, which have shaped our current standard of care approach to patients with advanced SCC of the larynx.
33.2 Case Discussion
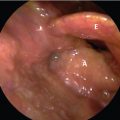
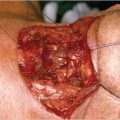
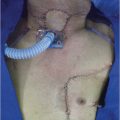
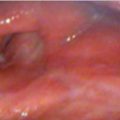
A 63-year-old male with a past history of hypertension presented to your clinic with a diagnosis of metastatic SCC of the larynx. The patient was found to have biopsy-proven SCC of the supraglottic larynx with metastasis to the lung and liver on imaging. He is hoarse and has some weight loss but otherwise is active and still working.
33.3 Targeting the Epidermal Growth Factor Receptor in the First-line Setting
The EGFR is a transmembrane receptor that is part of the ErbB family. Stimulation of EGFR leads to activation of downstream pathways including ras/raf/mitogen-activated protein kinase, phosphatidylinositol 3-kinase/v-Akt murine thymoma vial oncogene homolog, and phospholipase-C-y/protein kinase C, resulting in increased proliferation and survival of cells. 4 , 5 , 6 EGFR is overexpressed in approximately 90% of HNSCC patients and has been associated with a worse prognosis. 7 , 8 , 9 In SCC of the larynx, EGFR has been targeted with both monoclonal antibodies and tyrosine kinase inhibitors.
Cetuximab is an immunoglobulin G1 (IgG1) human-murine monoclonal antibody (mAb) that binds irreversibly to the extracellular domain of the EGFR receptor, blocking EGFR signaling. 10 It has been evaluated in the R/M setting in trials including laryngeal SCC both as a single agent and in combination with chemotherapy. Cetuximab was combined with platinum (cisplatin or carboplatin) and 5-FU in a phase III EXTREME Trial, comparing this regimen to platinum and 5-FU alone in the first-line setting for R/M HNSCC. Those in the experimental arm went on to receive cetuximab alone after six cycles of the triplet regimen. The addition of cetuximab led to a significant increase in response rate (RR; 36 vs. 20%; p < 0.001), progression-free survival (PFS; median: 5.6 vs. 3.3 months; hazard ratio [HR] for progression: 0.54; 95% confidence interval [CI]: 0.43–0.67; p < 0.001), and OS (median OS: 10.1 vs. 7.4 months; HR for death: 0.80; 95% CI: 0.64–0.99; p = 0.04), 11 resulting in its approval in combination with platinum and 5-FU by the Food and Drug Administration (FDA) and European Medical Agency in November 2011. It is currently the standard of care regimen for frontline treatment of R/M HNSCC including laryngeal SCC for patients who can tolerate a triplet regimen.
Panitumumab, a fully human IgG2 mAb against EGFR, was also combined with platinum and 5-FU in the phase III SPECTRUM trial. While the addition of panitumumab lead to a significant increase in RR and PFS compared to platinum and 5-FU alone, this did not translate into a significant OS benefit (median OS: 11·1 vs. 9.0 months; HR: 0·873; 95% CI: 0·729–1·046; p = 0.1403). 12 One possible reason for a lack of OS benefit with panitumumab as opposed to cetuximab is a longer OS in the control group in SPECTRUM compared to EXTREME (median OS: 9 vs. 7.4 months, respectively), which may have partially been from improved outcomes in patients from the Asia-Pacific geographic region (median OS of 11.7 months in control arm), which were not included in the EXTREME trial. Additionally, there are differences in the immunologic effects of the two antibodies. Cetuximab is an IgG1 mAb, and in contrast to IgG2 mAb panitumumab, cetuximab can induce the innate immune system through natural killer (NK) cell mediated antibody-dependent cellular cytotoxicity (ADCC) and additionally stimulate adaptive immunity, specifically the development of EGFR-specific CD8 T cells via NK cell/dendritic cell crosstalk. 13 , 14 Whether or not this contributed significantly to the difference in outcomes is not known.
33.4 Case Discussion (Continued)
Based on the EXTREME trial a regimen of cisplatin, 5FU and cetuximab is selected for treating this patient. The patient is treated with 6 cycles with a partial response and then is transitioned to cetuximab alone. Repeat imaging after 2 months of cetuximab alone shows progression of the cancers. The patient is started on nivolumab.
33.5 Targeted Therapy after Failure of Platinum-Based Chemotherapy
The patient has failed platinum-based chemotherapy. Traditional chemotherapeutic agents including taxanes, methotrexate, and gemcitabine have been evaluated in this setting with limited efficacy. 15 Treatment with cetuximab alone yielded a response rate of 13% and disease control rate of 46%; however, control was short lived as the time to progression was only 70 days. 16 Tyrosine kinase inhibitor afatinib, an irreversible ErbB family blocker, which inhibits EGFR, human epidermal growth factor receptor 2 (HER2), HER3, and HER4, was compared to methotrexate in a randomized phase III trial in HNSCC patients who had failed at least two cycles of platinum-based chemotherapy for R/M disease. Approximately 60% of patients had received prior mAb against EGFR in the frontline setting. PFS, the primary endpoint of the trial, was significantly increased with afatinib (median: 2.6 vs. 1.7 months; HR: 0.80; 95% CI: 0.65–0.98; p = 0.03). However, there was no difference in RR (10 vs. 6%; p = 0.10) or OS (median: 6.8 vs. 6 months; HR: 0.96; 95% CI: 0.77–1.19; p = 0.70) between afatinib and methotrexate. 17
33.5.1 Immune Checkpoint Inhibitors
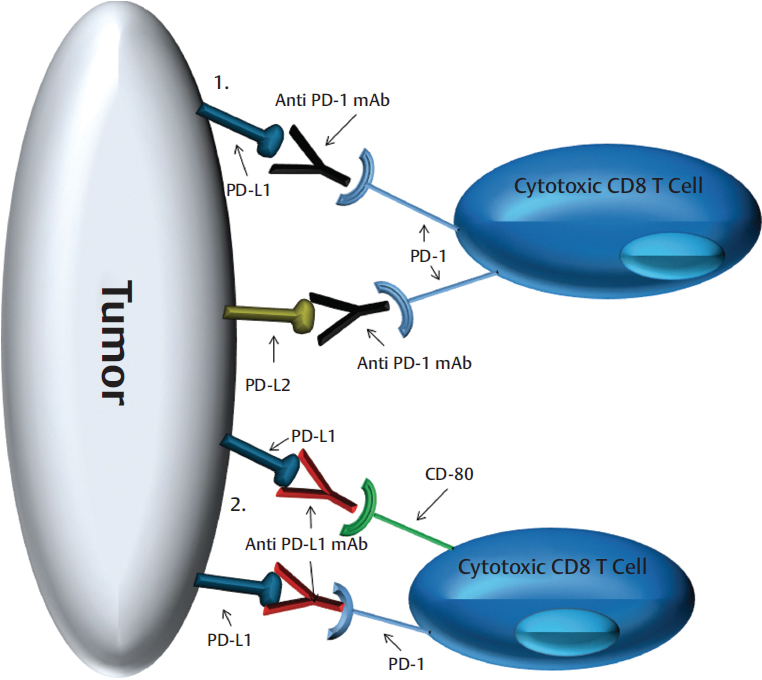
The immune response involves the complex interplay of immune cells driven by co-signaling molecule interaction and the cytokine/chemokine milieu. Immune checkpoint inhibitors target these co-signaling molecules and blockade of the PD-1:PD-L1 pathway has proven efficacious in multiple solid tumors including HNSCC. 18 , 19 , 20 Ligation of PD-1 on cytotoxic T cells by PD-L1 or PD-L2 expressed by tumor cells can result in T-cell anergy and apoptosis, providing protection for the tumor. 21 , 22 , 23 , 24 Anti-PD-1 mAbs block the interaction of PD-1 with PD-L1 and PD-L2, while anti-PD-L1 mAbs block the interaction of PD-L1 with PD-1 and CD80 ( Fig. 33‑1). Ligation of CD80 on effector T cells by PD-L1 can also lead to immune suppression. 25 Nivolumab, a fully human IgG4 anti-PD-1 mAb, was compared to investigators’ choice treatment (docetaxel, cetuximab, or methotrexate) in a randomized phase III trial in patients who had failed prior platinum chemotherapy. All patients were included regardless of PD-L1 status. Treatment with nivolumab was associated with a response rate of 13.3% and while there was no difference in PFS, nivolumab led to a significant increase in OS (median: 7.5 vs. 5.1 months; HR: 0.70; 97.73% CI: 0.51–0.96; p = 0.01). 26 This led to the approval of nivolumab in both the United States and Europe for R/M HNSCC, including laryngeal SCC, as standard of care treatment for patients with disease progression on or after platinum-based therapy, which includes failure within 6 months of platinum-based chemoradiation given in the upfront locally advanced setting. Another IgG4 anti-PD-1 mAb, pembrolizumab, was evaluated in a similarly designed phase III trial. The primary endpoint was OS in the intention to treat cohort with an efficacy boundary of one-sided α of 0.0175 corresponding to a HR for risk of death of 0.80. Initial analysis showed that patients treated with pembrolizumab had a median OS of 8.4 months compared to 7.1 months in the control arm (HR: 0.81; 95% CI: 0.66–0.99; p = 0.0204); therefore, the improvement in OS with pembrolizumab was not statistically significant. Interestingly, the control arm of Keynote 040 did better than in CheckMate 141 (7.1 vs. 5.1 months, respectively). This may have been partially driven by the receipt of subsequent immune checkpoint inhibitors by patients in the control arm. 27 However, updated analysis of Keynote 040, which included survival data on 11 additional patients, showed a decrease in the hazard ratio for the risk of death to 0.80 (0.65-0.98) with a p value of 0.0161 for the intention to treat cohort who received pembrolizumab. Anti-PD-L1 mAb durvalumab has been evaluated in phase II trials in platinum failure patients both alone and in combination with anti-CTLA4 (cytotoxic T-lymphocyte–associated antigen 4) mAb tremelimumab. In the single-arm phase II HAWK trial, which included R/M HNSCC patients who had failed platinum chemotherapy and were PD-L1 high (≥ 25% tumor cell PD-L1 expression), treatment with single agent durvalumab led to RR of 16.2% and a median OS of 7.1 months (95% CI: 4.9–9.9). 28 A phase III trial is ongoing evaluating durvalumab ± tremelimumab versus investigators’ choice chemotherapy in the platinum failure setting.
Treatment with anti-PD-1 or anti-PD-L1 mAbs can be associated with immune-related adverse events (IrAEs), which can affect any organ system including the pituitary (hypophysitis), thyroid (hypothyroid/hyperthyroid), skin (rash), lung (pneumonitis), liver (hepatitis), and colon (colitis). Importantly, these agents have been well tolerated in trials with, for example, less frequent G3/G4 treatment-related adverse events (13%) with nivolumab in CheckMate 141. Pneumonitis was only observed in 2.1% of patients. 26 However, IrAEs can occur at any time with these agents and the treating physician must remain vigilant as prompt treatment with steroids is required to prevent serious adverse events.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree