14 Transoral Robotic Surgery for Advanced Laryngeal Cancer
Abstract
Transoral robotic surgery (TORS) of glottic cancer was performed for the first time in 2009, and its usefulness in patients with laryngeal cancer has been reported. TORS is similar to transoral laser microsurgery in that it proceeds through the oral cavity, but it allows more precise surgery because two robotic arms can move freely within a narrow space. Application of TORS in treatment of laryngeal carcinoma has mainly been performed for early laryngeal cancer, but treatment results have also recently been reported for advanced laryngeal cancer. Induction chemotherapy was performed in patients with locally advanced laryngeal cancer to reduce tumor volume, and residual tumor was removed by TORS with excellent outcomes. These new treatment modalities are aimed at improving quality of life after treatment by preserving organ function. Based on pathologic information obtained after surgery in our trial, 20% of patients survived NED (no evidence of disease) without further radiation therapy, and the remaining patients received adjusted radiation doses based on pathologic adverse features. In these patients, we expect that long-term complications may be reduced by radiotherapy.
14.1 Introduction
In cases of locally advanced T3 and T4 laryngeal cancer, appropriate treatment methods are controversial because there is no reliable study comparing treatment outcomes of various treatment modalities. 1 T3 tumors have traditionally been treated with total laryngectomy as a single treatment modality, but partial laryngectomy is performed in some cases.s. Literatur Radiation (RT) alone has a 50% lower local control rate than surgery in T3 disease. 3 Accordingly, concurrent chemoradiotherapy (CRT) is recommended for conservative laryngeal surgery or for bulky T3 tumors rather than RT alone. T4 tumors usually undergo total laryngectomy, followed by adjuvant RT or CRT, and definitive CRT may be performed for long-term preservation in limited cases. Although nonsurgical organ preservation trials can be performed for long-term preservation with T4 tumors, preserved organs may not remain functional after RT. If edema persists 6 months after radiation, remaining or recurrent disease should be suspected. 4
The comparable oncologic outcome of transoral laser microsurgery has been reported only in cases of experienced operators with selective T3 and T4 tumors. 5 Park et al performed transoral robotic surgery (TORS) of glottic cancer for the first time in 2009 and reported its usefulness in patients with laryngeal cancer. 6 TORS is similar to transoral laser microsurgery in that it proceeds through the oral cavity, but it allows for precise surgery because two robotic arms can move freely within a narrow space. The use of TORS to treat laryngeal carcinoma has mainly been performed in early laryngeal cancer, but recently, treatment results have also been reported in advanced laryngeal cancer. 7 Induction chemotherapy was performed in patients with locally advanced laryngeal cancer to reduce tumor volume, and residual tumor was removed by TORS with excellent outcomes. 7 New treatment modalities are aimed at improving patient quality of life after treatment through preservation of organs and function.
14.2 Schematic Overview of Treatment Protocol (TORS Combined with Induction Chemotherapy)
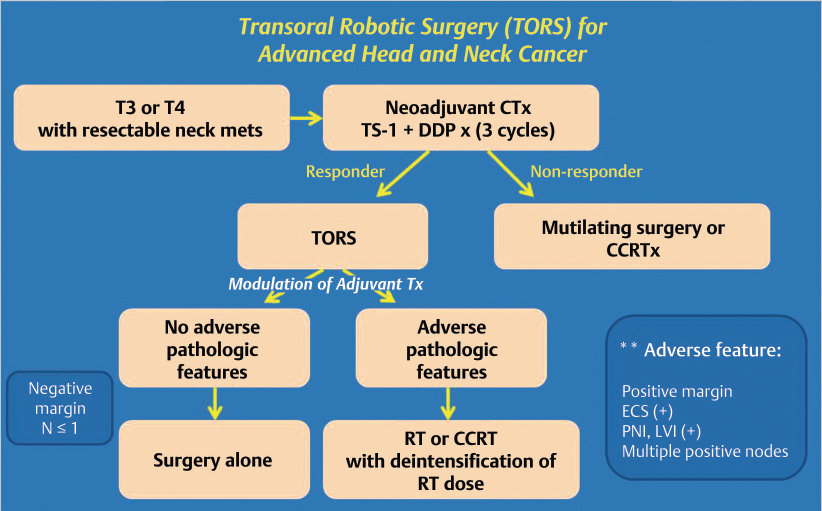
Patients with locally advanced laryngeal cancer and resectable nodal disease were considered for the treatment protocol. Induction chemotherapy was performed for three cycles before surgery, and TORS was performed in patients with more than partial response. Mutilating surgery or definitive concurrent chemoradiation therapy (CCRTx) was performed in patients without response to induction chemotherapy. In patients who received TORS, use of additional adjuvant chemotherapy was determined based on the results of pathologic examination of specimens obtained after surgery. In the case of pathologic N ≤ 1 with negative margins, treatment consisted of only surgery ( Fig. 14‑1).
14.3 Selection of Patients for Treatment Protocol
Indications for this treatment protocol are as follows: (1) patients with T3 or T4 laryngeal cancer who were histologically diagnosed with squamous cell carcinoma; (2) patients who had not undergone previous surgery, chemotherapy, or radiotherapy; and (3) patients with Eastern Cooperative Oncology Group performance status below 2. The following patients were considered to have relative contraindications: (1) patients who could not undergo radical resection of the lesion with TORS, (2) patients with distant metastasis, (3) patients who were not expected to have good follow-up compliance, and (4) patients with poor transoral exposure due to poor mouth opening.
14.4 Induction Chemotherapy Protocol
The chemotherapy regimen consisted of cisplatin (70 mg/m2) by intravenous infusion on day 1 and TS-1 (combination of gimeracil 5.8 mg/m2, oteracil 19.6 mg/m2, and tegafur 20 mg/m2) through oral route on days 1 through 14, which was repeated every 21 days. When myelosuppression appeared, chemotherapy was delayed for 1 week until leukocyte and platelet counts recovered. After two cycles of neoadjuvant chemotherapy, the response was evaluated by imaging studies and endoscopy based on Response Evaluation Criteria in Solid tumors. The largest tumor and neck mass diameter was measured to verify the response. TORS was performed on patients with more than partial response. Patients with less than partial response were considered for conventional surgery or definitive CCRTx ( Fig. 14‑1).
14.5 Operative Procedure
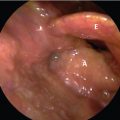
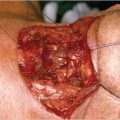
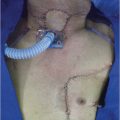
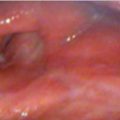
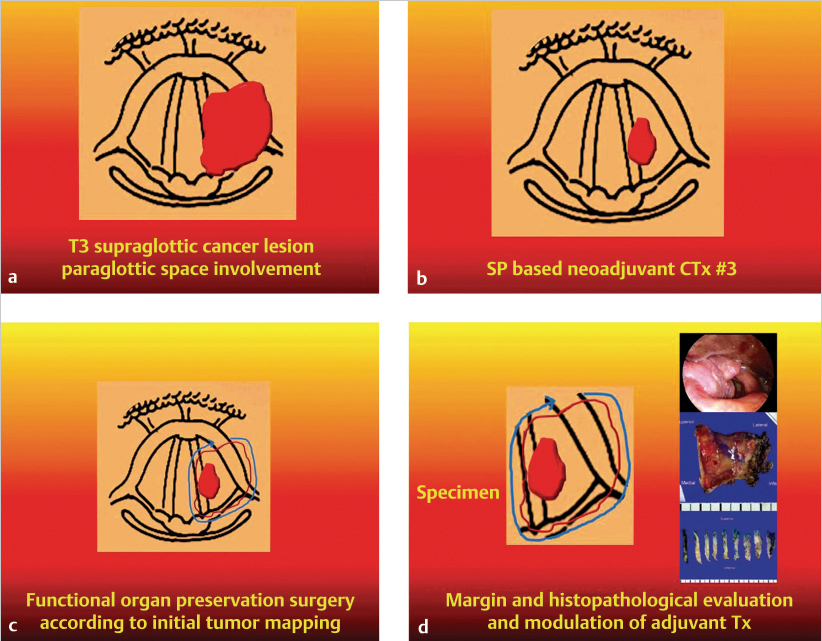
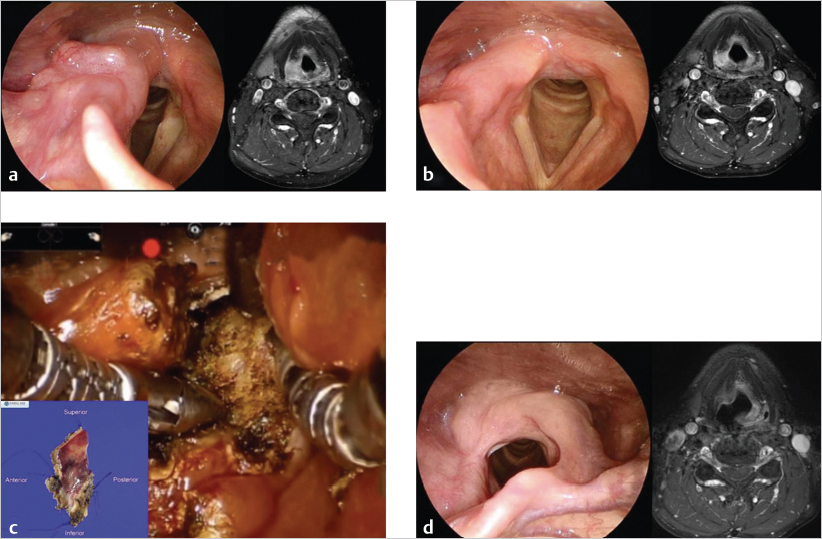
All patients underwent imaging studies, including CT, MRI, and PET, to assess the extent of disease. The extent of the primary lesion was recorded on video files captured via rigid or flexible endoscope. Based on the above data, the extent of resection was mapped based on the range of the pretreatment lesion rather than on reduced tumor volume after induction chemotherapy ( Fig. 14‑2). Case 1 was a 54-year-old man with T3N0M0 aryepiglottic fold cancer. The tumor measured 1.7 cm by MRI, and right vocal cord palsy was detected on rigid endoscopy. After induction chemotherapy, the tumor shrunk and vocal cord mobility recovered. The residual tumor was removed through TORS. On pathologic examination of the specimen, the surgical margin was negative and other adverse pathologic features were not observed. Therefore, the patient received surgery alone without radiotherapy, and there was no evidence of disease on the last visit ( Fig. 14‑3).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree