Fig. 19.1
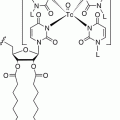
Structural similarities of adrenaline (upper molecule), noradrenaline (middle molecule), and 131I-mIBG (lower molecule)
19.1.2 mIBG: Uptake Mechanism
Meta-iodobenzylguanidine moves into cells by the mechanism of noradrenaline reuptake of the adrenergic presynaptic neuron, by binding to the noradrenaline transporter, a protein that is expressed in neuroendocrine tumors. It is concentrated in intracellular storage granules through vesicular monoamine transporters, as noradrenaline does, or remains in the cytoplasm (the latter is generally suggested to occur in NB). Unlike noradrenaline it shows no uptake by postsynaptic neurons and is not further metabolized. The main pathway of 131I-mIBG entry into the cells is via a Na+/ATP-dependent mechanism (uptake 1). Additionally, nonspecific low concentrations are achieved by passive diffusion (uptake 2) in the majority of cells. The half-life of 131I is at 8.04 days; beta maximum energy emission is at 0.61 MeV (average energy 0.192 MeV). Its mean range of penetration in soft tissues is approximately 0.5 mm. Therapy with 131I-mIBG is possible due to DNA damage and cancer cells’ death following their exposure to the radionuclide’s beta (β) irradiation [3]. The phenomenon of radiation-induced bystander effect might also contribute to cell death; however, its role in cytotoxicity is not well defined [4, 5].
19.1.3 Contraindications to 131I-mIBG Therapy
Absolute contraindications to 131I-mIBG therapeutic administration include pregnancy, lactation, less than 3 months’ life expectancy (except in cases of significant bone pain), and compromised renal function requiring dialysis at short intervals [6]. Relative contraindications to 131I-mIBG therapy include significant urinary incontinence, high medical risks from isolation, rapidly deteriorating renal impairment (GFR <30 ml/min), and bone marrow suppression (white blood cell count less than 3 x 109/L, platelet count less than 100 x 109/L). In the aforementioned conditions or in case of bone marrow invasion, the administered activity should be reduced.
19.1.4 Preparation of 131I-mIBG Therapeutic Administration
A prerequisite for therapy with 131I-mIBG is positive imaging with 123I-mIBG or 131I-mIBG in adults; the former is considered the radiopharmaceutical of choice for diagnosis. Thyroid blockade is necessary from 48 to 24 h before therapy until 10–15 days after therapy (usually with 130 mg potassium iodide in combination with 400 mg potassium perchlorate/24 h). Several drug classes (cardiovascular, sympathomimetics, antipsychotics, neuroleptics, tricyclic antidepressants, and CNS stimulants) that may affect the biokinetics of 131I-mIBG should be discontinued before treatment, if possible. In patients with metabolically active tumors secreting catecholamines taking alpha-adrenergic receptor antagonists to control blood pressure combined with beta-adrenergic receptor blockers for tachycardia control, withdrawal is not recommended due to the risk of complications, although the effectiveness of treatment might be diminished. Antiemetics (such as ondansetron) should be administered before treatment and for 3 days following treatment. Care must be given for complete and proper information to patients, and instructions (written and oral) should be delivered on the procedure before receiving treatment. Parents with proper guidance according to radiation safety protection can participate in the care of children receiving treatment [5]. Since more than 50 % of administered dose is excreted by the urine, a urinary catheter may be placed especially in children with NB to reduce bladder radiation dose and to avoid contamination.
19.1.5 131I-mIBG Administration Procedure
131I-mIBG is administrated in a therapy unit with lead shielding rooms via a slow iv infusion over 45 min up to 4 hours (usually over an hour to 2 hours); the procedure is undertaken by appropriately trained staff. Monitoring of vital signs is necessary, including blood pressure. The latter may be elevated, especially in patients with PHEO/PG [7]; this rise may be attributed to the release of noradrenaline from storage granules, possibly due to the infusion of substantial quantities of non-radiolabeled (or “cold”) mIBG (only one in 2000 molecules is labeled). In that case, short-acting alpha (followed by beta-)-adrenergic receptor blockers may be given, although usually infusion rate reduction or temporary interruption of the infusion is sufficient [5, 8]. Discharge of a patient from the special shielded room depends on national and local legislation, considering that the dose equivalent to persons with contact with the patient is insignificant (exposure rate <3 mrem/h at 1 m or in general 3–5 days).
19.1.6 Undesirable Effects of 131I-mIBG Therapy
Immediate effects include nausea, vomiting (usually limited to 2 days), and transient myelosuppression (4–6 weeks after 131I-mIBG administration); the latter is noted in children with NB after chemotherapy, and it is less common in adults receiving therapeutic 131I-mIBG. Rarely renal function can worsen, hypertensive crisis can ensue, or the parotid glands may get swollen (but no xerostomia is observed). Long-term undesirable effects of 131I-mIBG therapy include hypothyroidism (up to 25 % of patients, with lower incidence in adults), persistent bone marrow suppression, or the appearance of secondary neoplasias such as leukemia or myelodysplastic syndrome (<4 %) [9].
19.1.7 Myelosuppression
Myelosuppression is the most important undesirable effect of 131I-mIBG therapy [10]; the effect is more pronounced in thrombocytopenia vis-à-vis neutropenia, because platelets show selective 131I-mIBG uptake. In children with NB, bone marrow suppression peaks after 28 days after 131I-mIBG therapy, but may occur earlier if combined with chemotherapy. Myelosuppression from 131I-mIBG is dose dependent; it is mild at administered doses ≈ 7.4 GBq (200 mCi) in adults, while doses > 444 MBq/kg (12 mCi/Kg) may cause severe myelosuppression in both adults and children. Grade III or IV thrombocytopenia and/or neutropenia requires platelet transfusion and administration of hematopoietic growth factors [6].
19.1.8 131I-mIBG Dosing
Dosages of therapeutic 131I-mIBG are to their greater extent empirical. They vary depending on the patients, on toxicity (expected pretreatment and perceived posttreatment), on response to treatment, on disease progression rate, and on the accumulated experience of each center. In general, the therapeutic dose is usually 3.7–11.2 GBq (100–300 mCi). Dosing of 131I-mIBG can be fixed in amounts of limited toxicity (3.7–7.4 GBq; 200 mCi) or based on patient weight (96–666 MBq/kg) (2.6–18 mCi/kg) or body surface area (3.7–14.8 GBq/m2). Fixed doses are usually divided in multiple non-myelosuppressive segments over 3–6 months, based on response and toxicity. A high initial dose might be preferred for more responsive microscopic disease with rapid progression, whereas low repeated doses may be given to target macroscopic disease with slow progression. The doses can also be prescribed based on a dosimetric assessment of less than 2–4 Gy to total-body absorbed dose. If total-body radiation dose is higher than 2 Gy, provision for stem cells to be harvested before therapy (for autologous stem cell transplantation) should be made [6, 11–13].
19.1.9 131I-mIBG Dosimetry
The administered therapeutic 131I-mIBG dose is personalized, depending on disease burden, lesion distribution, and bone marrow reserves. Dosimetry with a pretreatment 123I-mIBG scan alone may underestimate the dose in 65 % of patients, but is more reproducible than that based on body weight. Posttreatment imaging is done to determine tumor targeting and its extent and retrospective dosimetry; due to high doses additional lesions may be detected in two-thirds of scans. Methods to estimate the total-body absorbed dose are either based on pretreatment dosimetry or prescribed as activity/kg, and posttreatment imaging is used to prescribe the second administration to achieve the goal of total-body dose. On the contrary to external beam radiation therapy, dosimetry of targeted radionuclide therapy may not be so precise, because of tumor volume variations and heterogeneous uptake by the targeted lesions. Nonetheless, total-body dosimetry is useful in predicting hematologic toxicity and assessing the radiation dose to tumor (usually cumulative doses of 60–150-Gy suffice), thus enabling individualized treatment [14–18].
19.2 131I-mIBG Therapy for PHEO/PG
Low (80–200 mCi; 2.96–7.4 GBq), intermediate (up to 500 mCi; 18.5 GBq), and high (>12 mCi/kg; >444 MBq/kg) doses have been used in PHEO/PG with cumulative dose up to 2,322 mCi (85,914 MBq). Patients that received a mean dose of 388 mCi (14.356 GBq) of 131I-mIBG and responded to treatment had a median survival of 4.7 years versus 1.7 for those without response to treatment. Furthermore, those who received over 500 mCi (18.5 GBq) as initial doses had a median survival of 3.8 years versus 2.6 years for those who received low doses [19]. In another study, patients who received intermediate doses of 269 mCi (9.953 GBq) compared to those with low doses of 149 mCi (5.513 GBq) had a quick response, similar survival rate, and mild side effects [20]. There is evidence that 131I-mIBG, given therapeutically at high activity and myelosuppressive doses (median dose 818 mCi or 12 mCi/kg, range 6–19 mCi/kg; 30.266 GBq, 444 MBq/kg, range 222–703 MBq/kg) after peripheral stem cell collection, may be associated with a higher response rate; the overall 5-year survival was 64 % compared to 44 % in patients without such therapy (based on a historical control group) [21]. At present the use of intermediate or high activities with autologous stem cell rescue is more advantageous due to the survival benefit.
Only approximately 0.05 % of 131I-mIBG molecules are radiolabeled, the rest being unlabeled mIBG (“cold carrier”). The latter is considered to competitively inhibit radiolabeled mIBG uptake by its target tissues, affecting treatment efficacy and may also provide adverse effects [22, 23]. In a phase I clinical study, 11 patients with refractory PHEO/PG were treated with high-purity and specific activity of no-carrier-added 131I-mIBG (radiolabeled 131I-mIBG more than 94 %; mean administered dose of 5.160 mCi (190.9 MBq). The study showed similar blood clearance and excretion characteristics to the carrier-added 131I-mIBG, and no side effects were related to the study drug [22].
There are no randomized trials or any consensus for the optimal strategy or of dosing schedules of 131I-mIBG therapeutic administration. Nor are there any comparative studies in patients given multiple non-myelosuppressive 131I-mIBG doses vis-à-vis high doses. Few prospective type studies of 131I-mIBG have been presented. Consequently, the observed differences in response to131I-mIBG treatment and survival could be attributed to patient selection. Several patients benefit from 131I-mIBG treatment: symptomatic response is high, ranging between 75 and 90 %, biochemical response rate is 45–74 %, and morphologic response is 27–47 % [24]. Nevertheless, complete response is low, ranging from 0 to 18 %, whereas 5-year survival ranges between 45 and 85 % [6, 12, 13]. The relative ease of the process, the partial response to treatment, the noted symptomatic and biochemical improvement, and limited side effects compared to chemotherapy make – regardless of its shortcomings – 131I-mIBG an attractive therapeutic option for patients with metastatic PHEO/PG.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree