Case study 113.1
A 52-year-old female was diagnosed with an incidental left renal mass discovered during evaluation for abdominal pain (see Figure 113.1).
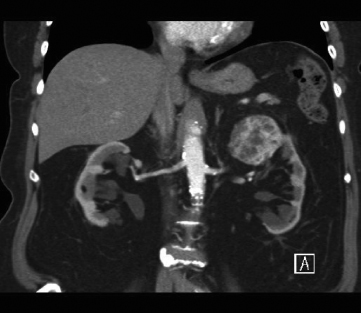 Figure 113.1 Left renal mass found in a 52-year-old female patient.
Figure 113.1 Left renal mass found in a 52-year-old female patient.
• What is the best imaging study for evaluation of a suspected renal mass?
Currently, computed tomography (CT) without and with intravenous contrast is the gold standard imaging study for the evaluation of a renal mass. Standard cross-sectional scans without and with contrast are sufficient to evaluate for mass enhancement, measured in Hounsfield units, and to evaluate vascular anatomy. Coronal and 3D reconstructions can also be useful for surgical planning by allowing for visualization of tumor complexity (nearness to collecting system or renal hilum), depth of penetration, and regional metastatic disease. Alternatively, magnetic resonance imaging (MRI) without and with gadolinium can be used with excellent anatomic detail, as well.
In patients with chronic kidney disease or a contrast allergy, CT and/or MRI can be performed without contrast. Suspicious renal lesions can be further characterized with ultrasound to better delineate solid or cystic components. However, cross-sectional imaging is essential for staging and for surgical planning.
• What is the likelihood of an enhancing renal mass being malignant?
The likelihood of malignancy increases with increasing renal mass size. Close to one-half of all renal masses less than 1 cm and approximately 20% of renal masses 2–4 cm in size are benign. Conversely, only about 6% of renal masses 7 cm or greater are benign. Additionally, high-grade tumors and negative prognostic features are more frequently identified in larger tumors. Other than fat-containing angiomyolipoma, current imaging modalities are unable to distinguish benign from malignant renal tumors.
• What is the role of renal mass biopsy for small renal masses?
Historically, renal mass biopsy was not routinely utilized in the management of small renal masses due to tissue inadequacy. This led to frequent inability to accurately assess tumor histology and grade. However, historical studies of renal mass biopsy utilized fine-needle aspiration (FNA). The current standard of renal mass biopsy involves utilization of core biopsy specimens (preferably, 18-gauge cores). Contemporary studies of renal mass biopsy with core biopsy specimens demonstrate diagnostic rates of greater than 80%. Additionally, concordance of renal mass biopsy with final surgical pathology was greater than 90%. Tumor heterogeneity and necrosis can affect accuracy rates for histology and grade on biopsy.
Indications for renal mass biopsy continue to change. Historically, biopsy was utilized to confirm metastatic disease to the kidney from a nonrenal primary malignancy or renal involvement of lymphoma. Currently, biopsy is frequently performed prior to ablative therapies and for further risk stratification in order to determine treatment or surveillance strategies.
In practice, renal mass biopsy is offered to all patients with an incidental renal mass. However, results of biopsy do not frequently alter treatment decisions of intervention or surveillance. This may be, in part, due to the low incidence of benign pathology (<20%) or, more commonly, patient preference.
• What are management options for small renal masses?
Management options include active surveillance, ablation, or surgical excision. Active surveillance has been recommended for some due to the slow growth rate of small renal masses (∼0.3 cm/year) and low risk of metastatic disease (<2%). Active surveillance of a small renal mass should be the initial management strategy for elderly patients with decreased life expectancy and those patients with extensive comorbidities considered high risk for intervention. Active surveillance is generally not recommended for younger patients or those with a longer life expectancy (>10 years) due to the small risk of progression. Unfortunately, the natural history of small renal masses has yet to be accurately elucidated, but disease progression to metastatic disease represents an incurable condition. Active surveillance generally involves serial imaging with CT, MRI, or ultrasound every 3 to 6 months initially. The interval between subsequent imaging studies can be varied based on observed growth rates. Intervention after an initial period of active surveillance still carries similar excellent oncologic outcomes as primary intervention.
Ablative therapies include cryoablation (CA) or radiofrequency ablation (RFA). These techniques represent minimally invasive treatment options for small renal masses. Each procedure can be performed via a percutaneous or laparoscopic approach and has the added benefit of treating the tumor in a nephron-sparing manner, thus potentially reducing the risk of long-term chronic kidney disease (CKD). Renal mass biopsy should be performed prior to ablation in order to guide future surveillance strategies.
Surveillance after ablation typically involves frequent cross-sectional imaging to evaluate for complete tumor ablation. However, there remains controversy regarding radiographic appearance post ablation and histologic success. Local recurrence has been shown to be higher for ablative techniques compared to surgical excision. Initial incomplete ablation of the target lesion is more common with percutaneous approaches, but repeat ablation can be performed. Surgical salvage of ablation failures can be quite challenging due to extensive perinephric fibrosis. Currently, ablative therapies are considered an option for masses smaller than 4 cm in patients considered higher risk for surgical intervention or in elderly patients. The risks of incomplete ablation and complications significantly increase in tumors larger than 4 cm. Ablation is generally not recommended for younger patients.
Surgical excision remains the gold standard management option for small renal masses. Specifically, partial nephrectomy has become the gold standard treatment. Oncologic outcomes are similar between partial and radical nephrectomy. However, there has been a significant accumulation of evidence that radical nephrectomy increases the risk of long-term chronic kidney disease when compared to partial nephrectomy. Evidence has also shown that CKD increases the risk of hospitalizations, cardiovascular events, and death as GFR decreases. Therefore, partial nephrectomy should be performed when technically feasible in order to preserve as much overall renal function as possible. Partial nephrectomy is an absolute indication in patients with preexisting CKD, bilateral renal masses, genetic predisposition (VHL, BHD, etc.), or solitary kidney.
Only one randomized controlled trial evaluating radical versus partial nephrectomy for small renal masses (<5 cm) has been performed. However, the study was terminated early due to poor accrual. Interestingly, overall survival was slightly lower in the nephron-sparing cohort versus the radical nephrectomy cohort (75.7% vs. 81.1%, respectively, at 9-year median follow-up). When patients with pathologically confirmed renal cell carcinoma (RCC) were analyzed, there was no difference in survival. Despite the findings of this study, the accumulation of other evidence suggests that partial nephrectomy should be the gold standard for management of small renal masses.
Partial or radical nephrectomy can be performed via open or laparoscopic approaches. More recently, robotic partial nephrectomy has become more common due to its shorter learning curve over laparoscopic partial nephrectomy. Partial nephrectomy does have a higher risk of perioperative complications compared to radical nephrectomy. Hemorrhage and urinary fistula are specific complications more commonly occurring after partial nephrectomy. Hemorrhage can occur at the time of surgery or present postoperatively as a delayed bleed, usually resulting from arterio-venous fistula or renal pseudoaneurysm. However, the rates of re-intervention after partial nephrectomy remain very low overall.
• How is renal function affected by intervention?
< div class='tao-gold-member'>



